Trump to Announce Auto Tariffs Wednesday
President Donald Trump indicated that auto tariffs were coming.

Steve Fossett: ‘The Adventurer’s Adventurer’
In this installment of ‘Profiles in History,’ we meet a commodities broker whose love of adventure established him as the greatest world-record holder.

‘Bob Trevino Likes It’: A Heartwarming Tale of Surrogate Fatherhood
‘Bob Trevino Likes It’ reminds us that valuable human relationships can occur randomly and that spiritual connections can be far more powerful than bloodlines.
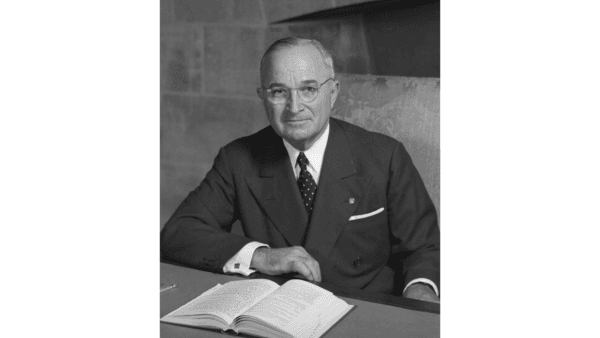
Ex Libris: Harry Truman
In this installment of our ‘Ex Libris’ series, we look at the books that influenced the last US president who guided the country through post-WWII changes.
Most Read
Top Stories
Intelligence Leaders Grilled by Democrats Over Handling of Military Information on Signal Chat
Gabbard suggested the chat was merely a policy discussion rather than a meaningful insight into U.S. military operations.
Russian Negotiators May Be ‘Dragging Their Feet’ in Ukraine Cease-Fire Talks, Trump Says
The Trump administration has negotiated a limited cease-fire covering Russia and Ukraine’s energy sites, but a broader truce is still in the works.
Reported Arrests of China’s Top Military Brass Indicate Political Crisis, Experts Say
Some analysts have said that the purge suggests that political infighting within the communist regime has intensified.
A Look at Trump’s Tariff Plan to Revive the US Auto Industry
Tariffs are a component in the toolkit Trump is using to achieve his goal of restoring domestic manufacturing, experts say.
EU Rejects Russian Demand to Lift Sanctions as Condition for Black Sea Cease-Fire
Zelenskyy said that Russia, not Ukraine, was holding up progress on a broader cease-fire.
Homeland Security Secretary Confirms Plans to Eliminate FEMA
Trump has signaled that he wants to either eliminate or overhaul the agency.
Dollar Tree Announces Sale of Family Dollar Subsidiary for $1 Billion
Dollar Tree shares have tanked by more than 44 percent over the past year.
Treasury Department Planning to Fire ‘Substantial’ Number of Workers: Official
The exact numbers were not disclosed.
Congress Risks August Default Without Debt-Ceiling Action: Congressional Budget Office
The latest forecast is ‘a clear warning sign,’ the Committee for a Responsible Federal Budget said.
International Student at Tufts University Detained by Immigration Authorities
The university said it would help the student obtain outside legal resources if requested.
▶Shen Yun is a Reminder of ‘How Much Good’ There Is in the World
Shen Yun Performing Arts brought the rich cultural heritage of ancient China to life at a sold-out performance at the State Theatre New Jersey in New Brunswick.
US Pauses Some Green Card Applications for More Vetting
The agencies said the new directive is to align with Trump’s Jan. 20 executive order.
US Blacklists Dozens of Chinese Companies Over National Security Concerns
‘We will not allow adversaries to exploit American technology to bolster their own militaries and threaten American lives,’ Commerce Secretary Lutnick said.
Tracking Trump’s High Level Appointments, Senate Confirmations
The Senate is undertaking the confirmation process for the president’s new administration.
GameStop to Close Hundreds of Stores, Pursue Bitcoin Investment Strategy
GameStop plans to shutter a significant number of its stores and invest in bitcoin as a treasury reserve asset, the company disclosed in its latest SEC filings.
US Attorney Jessica Aber’s Likely Cause of Death Revealed by Police in Virginia
Officials say that Aber likely died of natural causes. Neither the specific cause nor manner of death have been released.
New Messages From Government Signal Group Released; White House Responds
The Atlantic obtained the messages.
Supreme Court Upholds Biden-Era ‘Ghost Gun’ Regulation
The regulation had been challenged as a misinterpretation of federal law.
Injuries From Recalled Products Hit Highest Level in 8 Years
Sourcing from China was a key reason that the Consumer Product Safety Commission has been issuing significantly more product warnings, an agency official said.
Judge Reverses Decision, Says Unions Can Sue Over Federal Worker Firings
The judge said he reconsidered his decision in light of additional briefing.
Belvoir Castle: A Gothic Revival Masterpiece
In this installment of ‘Larger Than Life: Architecture Through the Ages,’ we visit a faux historic castle in Leicestershire, England.
Special Coverage
Special Coverage