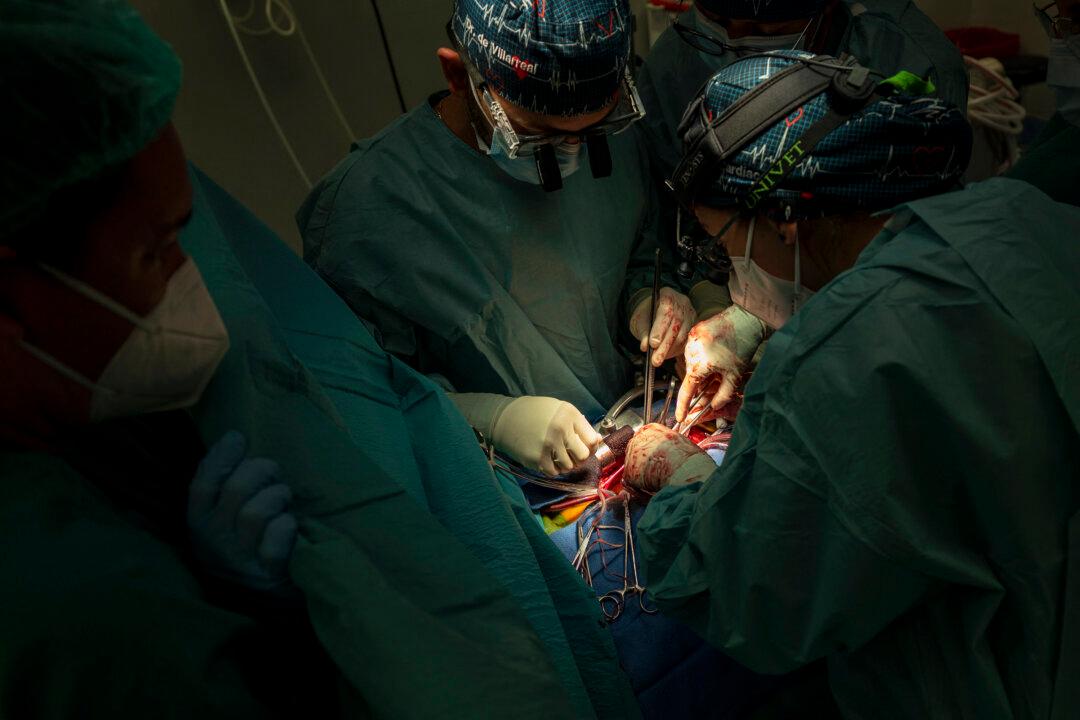
Researchers from the Murdoch’s Children Research Institute (MCRI) are developing new treatments for congenital heart disease that could enable children born with birth defects can regenerate the damaged organ.
In 2011, Prof. Enzo Porrello, who is now head of the Heart Regeneration Laboratory at the MCRI, demonstrated the regenerative properties of newborn mouse hearts at the University of Texas Southwestern Medical Centre. Prior to this research, the capacity of mammalian hearts to regenerate was a debated topic.