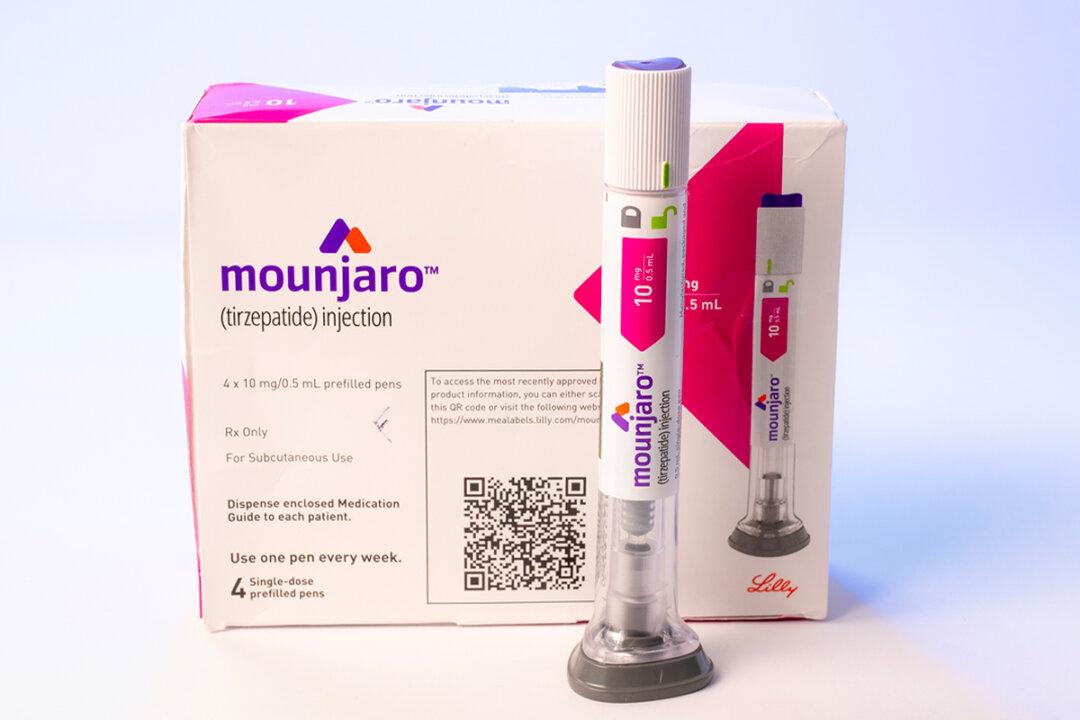
A diabetes drug Mounjaro (tirzepatide), has been approved by an executive medicines regulator to treat obesity in adults aged 18 and over.
The Medicines and Healthcare products Regulatory Agency (MHRA) authorized the use of Mounjaro for weight management and weight loss. Patients, who take the medicine, are supposed to follow a reduced-calorie diet and exercise actively.