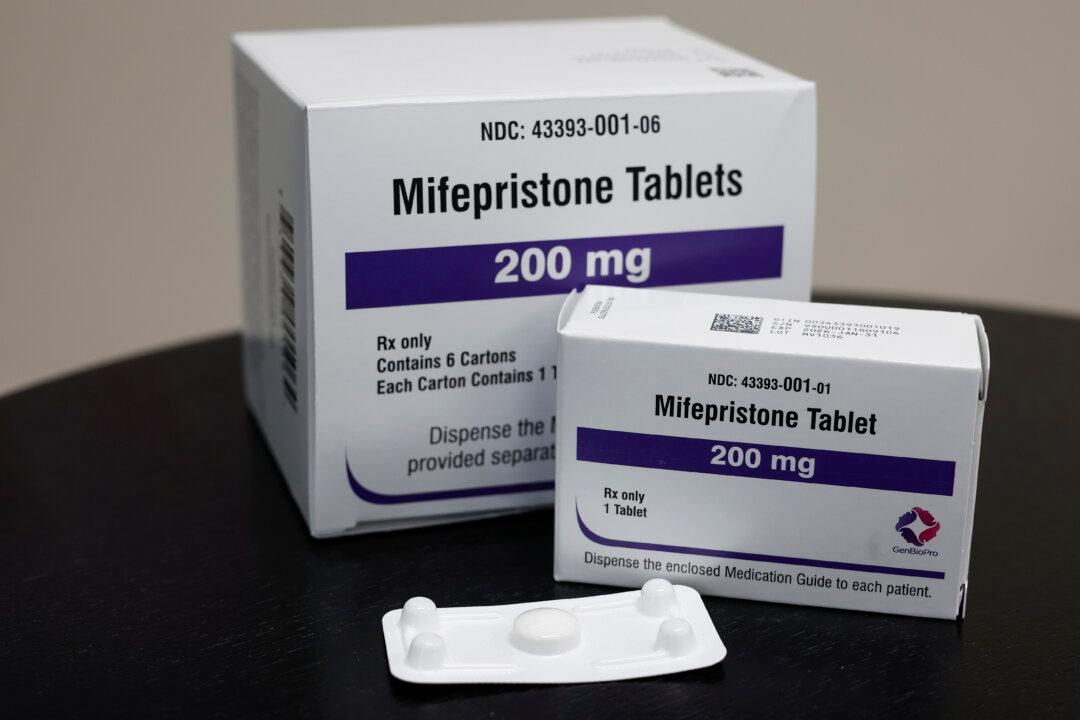
Republican attorneys general in three states—Idaho, Kansas, and Missouri—have sued several Biden administration agencies, alleging that they unlawfully approved shipment of dangerous abortion drugs by mail while rolling back key safety precautions, exposing women and girls who get chemical abortions to potential harm.
Missouri Attorney General Andrew Bailey said Monday that his office has filed a lawsuit against the Food and Drug Administration (FDA) and the U.S. Department of Health and Human Services (HHS) over the agencies’ illegal approval of shipments of chemical abortion drugs (mifepristone and misoprostol) by mail across the United States.