Focus
Pfizer
LATEST
‘Stay Home’: Western Australia Issues COVID-19 Warning Ahead of Christmas
Dr. Paul Armstrong suggested residents consider wearing a mask in crowded settings
|
Local WA Council Votes to Suspend Pfizer and Moderna COVID-19 Vaccinations
The local mayor and premier of Western Australia suggested the council should be focussed on local issues.
|
UK Watchdog Accuses Pfizer of Promoting ‘Unlicensed’ COVID Vaccine on Social Media
The PMCPA ruled that Pfizer has breached its regulatory code, including ‘bringing discredit’ on the pharmaceutical industry.
|
Court Finds Emergency Doctor Guilty of Misconduct for Questioning COVID Vaccine
The Western Australian doctor said the vaccine was ‘at best manslaughter, and at worst, outright murder.’
|
New Bill Would Strip COVID-19 Vaccine Manufacturers of Liability Protection
Rep. Chip Roy said Americans ‘deserve justice for the infringement on their personal medical freedom and those medically harmed deserve restitution.’
|
Pfizer Filing Shows 70 Percent Decline in COVID Vaccine Revenue
Pfizer wrote in an SEC filing that its vaccine revenue is down 70 percent for 2023.
|
Frequent COVID Boosters in Immunocompromised ‘May Be Causing More Harm Than Benefit,’ New Review Suggests
Repeated mRNA COVID-19 vaccination impairs the activation of immune cells that protect the body from infections and cancer.
|
Denied and Attacked: Vaccine-Injured Health Care Workers Demand to Be Heard, Acknowledged, Compensated
Health care workers share their stories of being injured by COVID-19 vaccines.
|
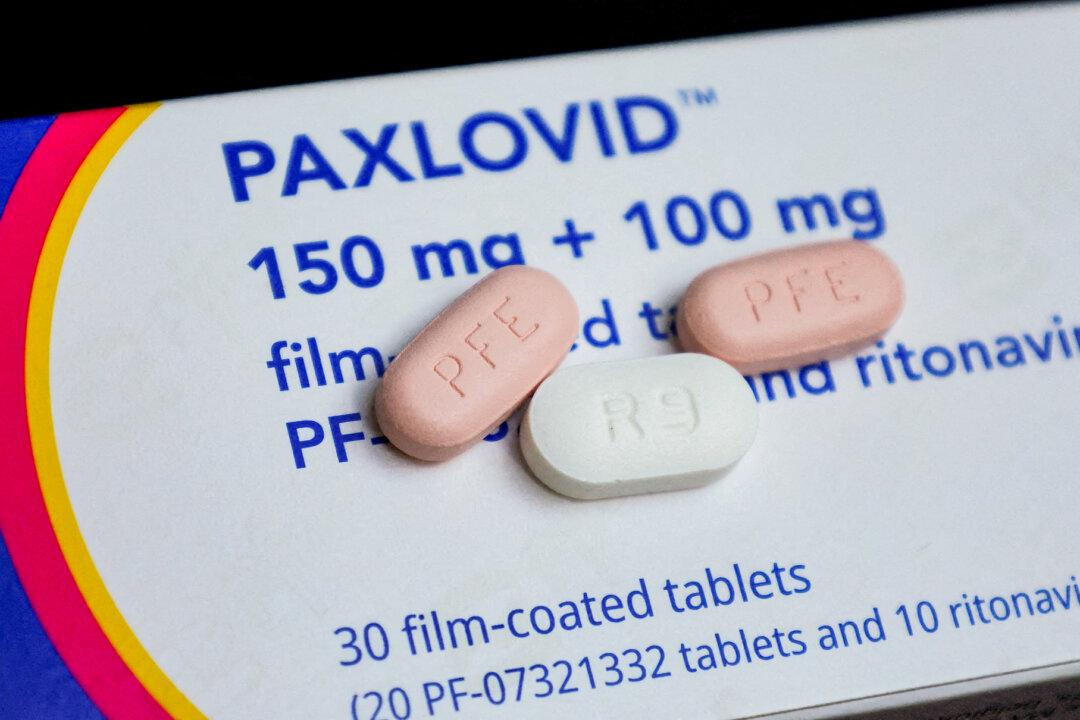
FDA Revises Emergency Use of Paxlovid
The revision is a step toward finalizing the transition from Paxlovid’s emergency use authorization to its new drug application label, initiated in late 2023.
|
Majority of Americans Believe COVID Shots Cause ‘Unexplained Deaths’
About a quarter of American adults said they ‘personally know someone’ who they believe was killed by COVID-19 vaccine-related side effects, a survey shows.
|
‘Stay Home’: Western Australia Issues COVID-19 Warning Ahead of Christmas
Dr. Paul Armstrong suggested residents consider wearing a mask in crowded settings
|
Local WA Council Votes to Suspend Pfizer and Moderna COVID-19 Vaccinations
The local mayor and premier of Western Australia suggested the council should be focussed on local issues.
|
UK Watchdog Accuses Pfizer of Promoting ‘Unlicensed’ COVID Vaccine on Social Media
The PMCPA ruled that Pfizer has breached its regulatory code, including ‘bringing discredit’ on the pharmaceutical industry.
|
Court Finds Emergency Doctor Guilty of Misconduct for Questioning COVID Vaccine
The Western Australian doctor said the vaccine was ‘at best manslaughter, and at worst, outright murder.’
|
New Bill Would Strip COVID-19 Vaccine Manufacturers of Liability Protection
Rep. Chip Roy said Americans ‘deserve justice for the infringement on their personal medical freedom and those medically harmed deserve restitution.’
|
Pfizer Filing Shows 70 Percent Decline in COVID Vaccine Revenue
Pfizer wrote in an SEC filing that its vaccine revenue is down 70 percent for 2023.
|
Frequent COVID Boosters in Immunocompromised ‘May Be Causing More Harm Than Benefit,’ New Review Suggests
Repeated mRNA COVID-19 vaccination impairs the activation of immune cells that protect the body from infections and cancer.
|
Denied and Attacked: Vaccine-Injured Health Care Workers Demand to Be Heard, Acknowledged, Compensated
Health care workers share their stories of being injured by COVID-19 vaccines.
|
FDA Revises Emergency Use of Paxlovid
The revision is a step toward finalizing the transition from Paxlovid’s emergency use authorization to its new drug application label, initiated in late 2023.
|
Majority of Americans Believe COVID Shots Cause ‘Unexplained Deaths’
About a quarter of American adults said they ‘personally know someone’ who they believe was killed by COVID-19 vaccine-related side effects, a survey shows.
|