Focus
Paxlovid
LATEST
FDA Revises Emergency Use of Paxlovid
The revision is a step toward finalizing the transition from Paxlovid’s emergency use authorization to its new drug application label, initiated in late 2023.
|
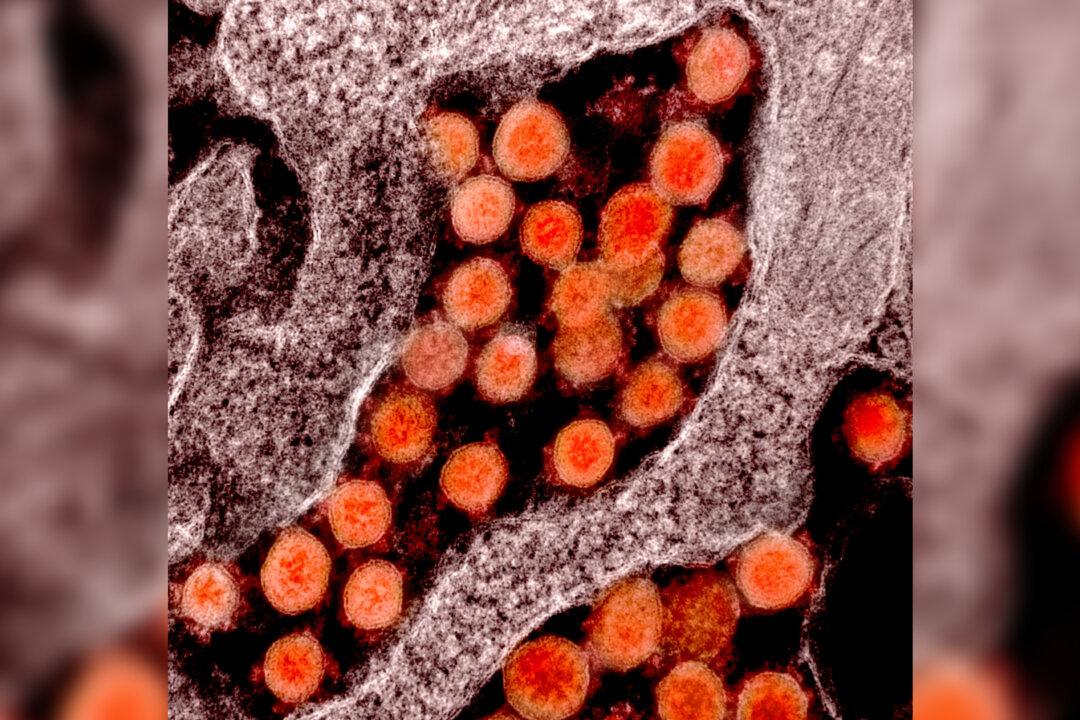
How the COVID Virus Defends Itself From the Human Immune System
Researchers believe they’ve identified the mechanisms that allow the SARS-CoV-2 virus to be so effective at making us sick.
|
Paxlovid Doesn’t Reduce Risk of Long COVID, Potentially Linked to Rebound Symptoms: Study
Researchers found little difference in outcomes between Paxlovid users and nonusers. They also found 1 in 5 users experienced rebound symptoms.
|
FDA Approved Nearly 50 Percent More Drugs in 2023 Than in 2022
Only 2018 featured a higher number of drug approvals.
|
One in Five Pfizer Drug Users Experience COVID-19 Rebound: Study
Many Paxlovid recipients have suffered from renewed symptoms.
|
Paxlovid Does Not Work Against ‘Long COVID’: Study
Out of the 31 long COVID conditions, Paxlovid was found to minimize the incidence of only one.
|
Pfizer to Charge Nearly $1,400 for COVID-19 Drug Paxlovid
Pfizer projects Paxlovid sales to fall by 58 percent this year compared to 2022.
|
Study Finds Paxlovid, Molnupiravir Reduce Omicron Hospitalization, Mortality
Researchers said that both oral antiviral drugs can be used to treat nonhospitalized patients at high risk of progressing to severe COVID-19.
|
FDA Revises Emergency Use of Paxlovid
The revision is a step toward finalizing the transition from Paxlovid’s emergency use authorization to its new drug application label, initiated in late 2023.
|
How the COVID Virus Defends Itself From the Human Immune System
Researchers believe they’ve identified the mechanisms that allow the SARS-CoV-2 virus to be so effective at making us sick.
|
Paxlovid Doesn’t Reduce Risk of Long COVID, Potentially Linked to Rebound Symptoms: Study
Researchers found little difference in outcomes between Paxlovid users and nonusers. They also found 1 in 5 users experienced rebound symptoms.
|
FDA Approved Nearly 50 Percent More Drugs in 2023 Than in 2022
Only 2018 featured a higher number of drug approvals.
|
One in Five Pfizer Drug Users Experience COVID-19 Rebound: Study
Many Paxlovid recipients have suffered from renewed symptoms.
|
Paxlovid Does Not Work Against ‘Long COVID’: Study
Out of the 31 long COVID conditions, Paxlovid was found to minimize the incidence of only one.
|
Pfizer to Charge Nearly $1,400 for COVID-19 Drug Paxlovid
Pfizer projects Paxlovid sales to fall by 58 percent this year compared to 2022.
|
Study Finds Paxlovid, Molnupiravir Reduce Omicron Hospitalization, Mortality
Researchers said that both oral antiviral drugs can be used to treat nonhospitalized patients at high risk of progressing to severe COVID-19.
|