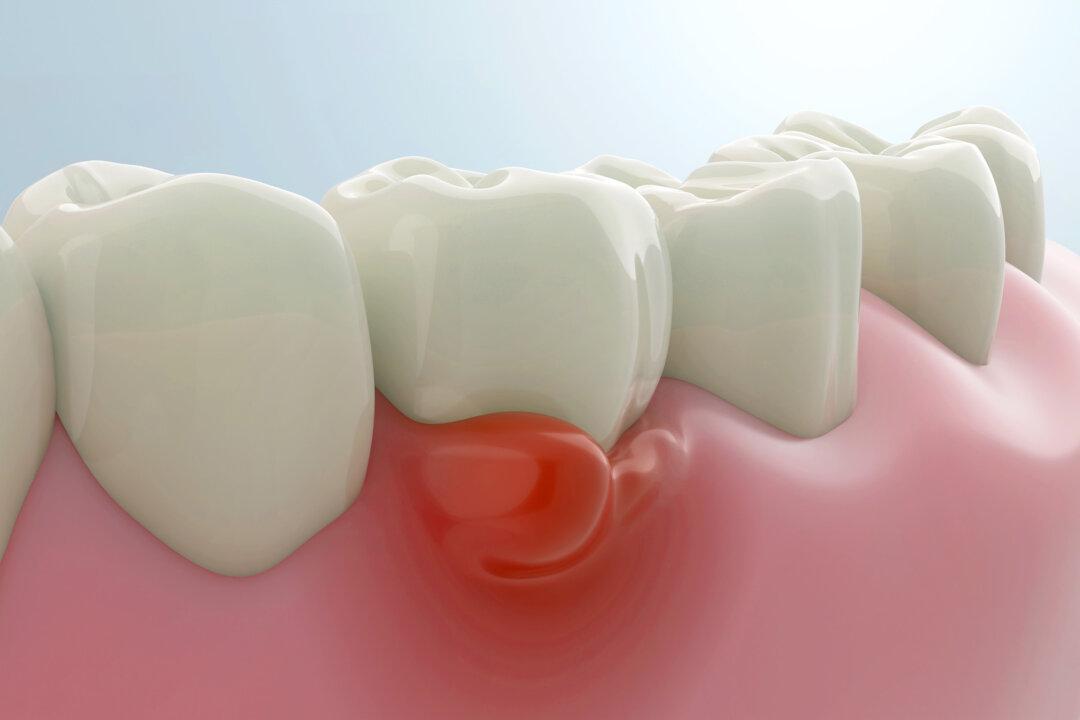
Scientists have found a new antibiotic that targets the main culprit behind gum disease.
In an August study published in the Journal of Oral Microbiology, researchers found that a narrow-spectrum antibiotic, FP-100, eliminated bad bacteria called Fusobacterium nucleatum in mice—without harming beneficial bacteria—and “significantly” reduced bone loss that had resulted from the condition.