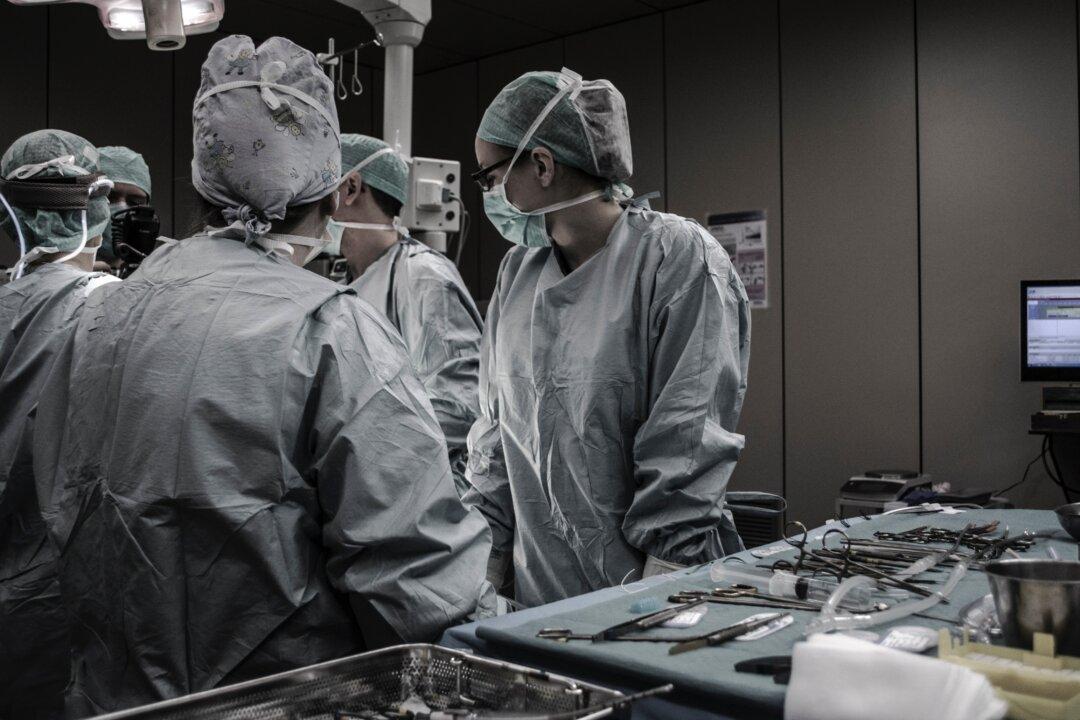
But beyond just this example, what does happen when scientists fail to comply with globally-accepted guidelines for ethical medical research? We examined this issue focusing on published research involving recipients of organ transplants performed in the People’s Republic of China.
- involves any biological material from executed prisoners
- lacks human research ethics committee approval
- lacks consent of donors.
Human organ transplants in China
Using a scoping review methodology, we examined 445 studies published in peer-reviewed English language journals between January 2000 and April 2017. The papers reported research involving recipients of human organ transplants (limited to hearts, livers or lungs) taking place in China. The data included 85,477 transplants.We found that 92.5 percent of the publications failed to state whether or not the transplanted organs were obtained from executed prisoners. Nearly all of them (99 percent) failed to report whether organ donors gave consent. In contrast, 73 percent of papers reported approval from an institutional ethics committee for the research reported in the paper.
Where do the organs come from?
In 19 papers involving 2,688 organs transplanted prior to 2010, the source of organs was reported as voluntary donors. But as widely known in the transplantation community, there was no volunteer deceased organ donor program in China before a pilot started in 2010. It therefore seems reasonable to assume that organs may have been sourced from prisoners, making claims about volunteer donation unreliable.The two journals that published the greatest number of Chinese transplant papers identified in our study are Transplantation Proceedings, with 65 of the total 445 papers, and PLOS ONE, with 20. Other journals with papers identified by this study include the American Journal of Transplantation, and Transplantation (the official journal of the peak international body, The Transplantation Society). These journals both have policies that explicitly prohibit the publication of research based on organ transplants from non-consenting and/or prisoner donors.
We argue that, if they are not already doing so, reviewers and medical journals should demand information about the source of organs in Chinese transplant research before these are published for the broader public and scientific communities. If they are demanding such information, the responses to those demands should be published. And if they are not satisfied with the responses, they should refuse to publish the research.
We’re all responsible
Our findings raise important and disturbing questions about ethical oversight on the part of all of those involved in the process of reviewing and publishing transplantation research.In response, we propose large-scale retractions of the papers identified by our research that are not consistent with international standards regarding organ donation.
We also propose a moratorium on all clinical transplant publications from China pending an international summit. The summit of transplantation community members and other stakeholders could develop appropriate policies and processes for handling future research.

As for the authors involved in retracted or ethically non-compliant research, there is little information about the impact on their careers. Being the author on a paper that is retracted may lead to a ban by the journal, trigger an institutional investigation, or have no effect.