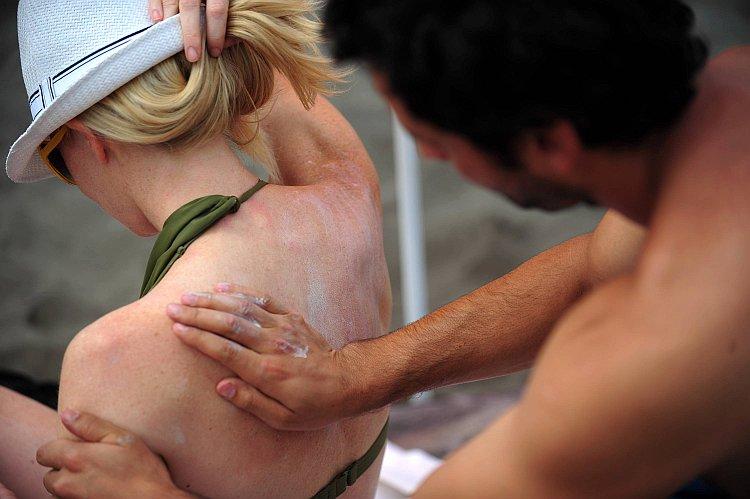
Summer heat means more sun protection, but how can you tell if the product you buy actually does the job? New sunscreen standards that were supposed to go into effect next month are being held back until December by federal regulators.
According to the American Academy of Dermatology, unprotected sun exposure is the most preventable risk factor for skin cancer. But because regulators have long classified sunscreens as a cosmetic, these products have largely avoided government oversight, making it difficult for consumers to determine which sunscreens work as they claim.
With skin cancer on the rise, the call for better testing and label requirements has grown louder. After years of deliberation, last June the U.S. Food and Drug Administration (FDA) announced new regulations designed to distinguish genuine protection from empty promises.
“We want consumers to understand that not all sunscreens are created equal,” says Lydia Velazquez, of the FDA’s Division of Nonprescription Regulation Development.
In a statement, Velazquez says the new guidelines “will help consumers know which products offer the best protection,” but for now they remain a mystery. On May 11, the FDA announced that they need to wait a little more.
Since 1978, the FDA has been considering regulations targeting inaccurate sun-protection claims, and critics say they can’t afford to stall any longer. Legislators and consumer advocacy groups are trying to convince the agency to stick with the original timeline.
Leading the charge is Senator Jack Reed (D-R.I.), who says the FDA needs “to stop dragging their feet” and give “consumers the protection they deserve.”
Sun protection has been an important issue for Sen. Reed, author of the Sunscreen Labeling Protection Act. In a recent letter, Reed and other Senate Democrats called the decision to delay regulations “a major step backward” and strongly urged the FDA to reverse it.
Lawmakers say regulations that would have gone into effect on June 18 were “a positive signal to Americans that they would not go another summer without critical protection from the sun. Unfortunately, delaying the implementation of these standards by six months (for some manufacturers, and 18 months for others) will allow the deceptive practices of the industry to continue.”
The new guidelines are sure to have an effect on the sunscreen market, and the FDA says they are postponing new rules to “avert a shortage.” But according to the Environmental Working Group (EWG), regulators are simply caving to industry pressure. The consumer advocate says the Personal Care Products Council and the Consumer Healthcare Products Association are the real reason behind regulation delay.
Not that the EWG has been all that impressed with the new guidelines. The group previously accused federal regulators of submitting to industry demands when the FDA released their “low-bar” new rules last year.
EWG estimates that 90 percent of sunscreens on the market are already in compliance with what it calls the FDA’s “weak” regulations on efficacy and safety. For example, the groups says new rules will allow most products on the U.S. market to use the label ‘broad spectrum sunscreen,’ even though “some will not offer enough protection to assure Americans they can stay in the sun without suffering skin damage from invisible UVA radiation.”
Contrast this with regulations seen elsewhere. According to the EWG, about 20 percent of products that meet the new FDA rules wouldn’t make the cut in Europe, where UVA standards are strict by comparison.
The Epoch Times publishes in 35 countries and in 19 languages. Subscribe to our e-newsletter.