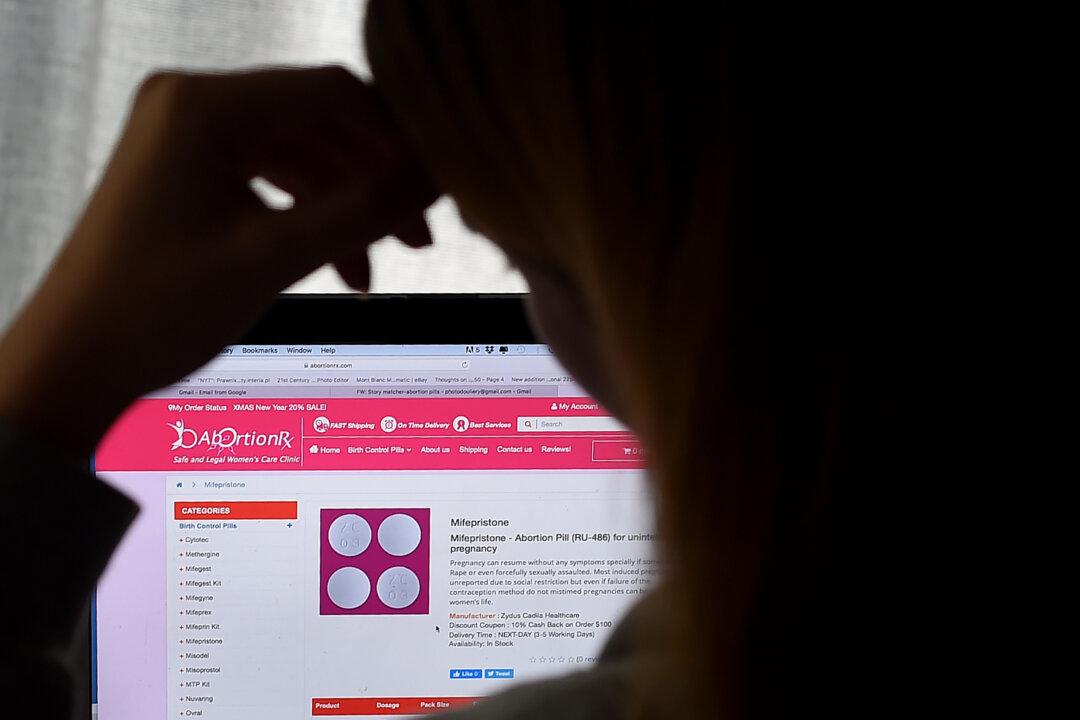
Democrats and Republicans are squaring off in dueling legal efforts in opposition and defense of the widely used abortion pill.
Dozens of attorneys general from both red and blue states are taking sides in a battle that will ultimately determine the fate and availability of the widely used abortion pill.