A patient alert system was announced on Monday by the medical regulator to monitor men taking the drug finasteride, which can cause serious psychiatric problems and interfere with normal sexual function even after the medication has been stopped.
The Medicines and Healthcare products Regulatory Agency (MHRA) has no plans to withdraw the drug and is instead urging men to “stay vigilant” for side effects following its review into the safety of finasteride—used by the NHS to treat an enlarged prostate and often prescribed privately to combat male pattern baldness.
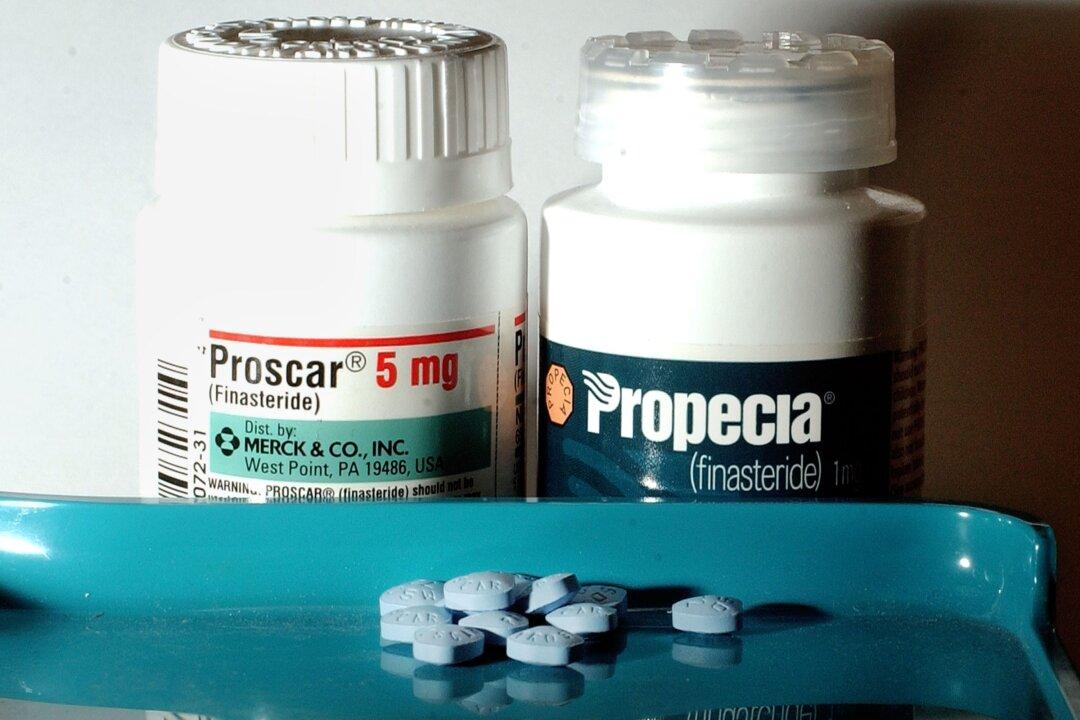
Propecia, the brand name for the version of finasteride used to treat hair loss, and Proscar, used to treat enlarged prostate, are manufactured by Organon, a spinoff from the pharma giant Merck & Co. Other companies have been making a generic version of finasteride since 2014, when the patent for Propecia expired.
“Since the first report was received in November 1992, the MHRA has received 426 Yellow Card reports up until 5 April 2024 of finasteride both 1mg [for hair loss] and 5mg [for prostate] formulations) and sexual dysfunction, including reports of erectile dysfunction (inability to get and maintain an erection) and decreased sex drive.”
The regulator adds that in almost half of these reports, the outcome was recorded as “not recovered” or “not resolved.”
‘Post-Finasteride Syndrome’
Campaigners have been calling for the drug to be more strictly regulated and for patients to be made aware of the potentially serious side effects, with one man telling The Epoch Times how he has suffered a “systemic destruction in every aspect” of his health, since he took the pills in the 1990s.Ryan Clark said that through an online forum, he had identified a drug-specific condition known as post-finasteride syndrome, brought on by the hair loss drug, which seemed to cause some men to have life-long debilitating illness and suicidal thoughts.
He and other campaigners are concerned about the ease with which the drug can be obtained through private prescription from online chemists, including Superdrug and Lloyds Pharmacy, and the way in which it is marketed on social media to younger men who are self-conscious about hair loss.
Mr. Clark said he welcomes the review and the new warning system from the MHRA, but is worried there could be a “generation” of young men who have harmed themselves through the drug, which could be a “ticking time bomb” of health problems.
He took the drug from his early 30s, and was not warned of any possible side-effects. He took finasteride on and off for many years, until he eventually made the connection between the medication and the terrible health problems he experienced, which doctors had been unable to help him with.
He believes there are many more symptoms that may be caused by the drug than the review has identified.
His wide-ranging symptoms included his face shape gradually changing—not in a typical age-related way, but “feminizing” in shape—while his skin stopped producing oil and his gums receded, his vision blurred intermittently, his urination changed, and his digestion was destroyed.
He believes that suicides in people suffering from the syndrome—not recognised by the NHS but acknowledged in specialist research papers—were being incorrectly explained away as being down to “depression” in men over losing their hair, when it could have been down to the hormonal disruption caused by the drug.
“I was never depressed about going bald. I was just a vain guy, I wanted to look my best, and I thought this was a risk-free solution,” he said.
He said that men suffering from the syndrome all report a loss of masculinity through a changed jawline and shrinking genitalia as well as a diminished libido. He believes the drug appears to cause fewer problems when given for enlarged prostate because the men being treated are generally older.

‘Patients Should Use Yellow Card System’
The regulator said in a statement: “As a recommendation of the review, a patient alert card will be introduced into the finasteride pack this year to raise awareness of these risks and help to ensure that patients are informed on what to do if they experience these side effects.“As patients may not notice changes to their mood, they are encouraged to show this card, and the patient leaflet, to friends and family.”
The MHRA issued a previous alert after carrying out a review into the drug in 2017, but said it was not “widely known” then that the side-effects could persist for months or even indefinitely after the medication was stopped.
The regulator said that men who are seeking treatment privately for hair loss should inform their prescriber of any personal history of depression. If someone taking Propecia for hair loss develops depression or suicidal thoughts, they should “immediately” stop their medication and contact their doctor.
If men are taking the drug for benign enlargement of the prostate and develop depressive symptoms, they should speak to their doctor urgently for advice, the MHRA said.
The regulator added that if patients experience problems with sexual function, they should discuss this with their prescriber or doctor.
The MHRA said it had reviewed the available evidence, including Yellow Card reports, published scientific literature and the actions of other regulators, and this was considered by the Pharmacovigilance Expert Advisory Group (PEAG) of the Commission on Human Medicines.
“The PEAG noted that the product information for finasteride contains information regarding the potential for persistent sexual side effects after discontinuation with finasteride and depression and suicidal ideation. However, these side effects are not well known by prescribers and patients and therefore a Drug Safety Update article was recommended,” the regulator said.
Drug Developed From Study of Hermaphrodite Population
The medication was developed from a 1970s study in the Dominican Republic of “Guevedoces,” which looked at children born with ambiguous genitalia and raised as female until puberty, at which time they began to develop male genitalia.The study found the deficiency of the 5-alpha reductase enzyme in the “Guevedoces” showed an inability to convert testosterone into DHT. Merck later picked up this study initially to create the medication designed to address enlarged prostate, a condition prevalent in middle-aged and older men which can cause the need for frequent urination and problems emptying the bladder.
Finasteride later found its way into the cosmetic drug market after it was discovered to trigger hair growth as a side-effect among users of the prostate medication.
It adds that sometimes finasteride “can cause a rash, reduced sex drive, low mood, erection problems or tenderness on or around the nipples. Very rarely, sexual side effects can be permanent and remain after stopping the medication.”