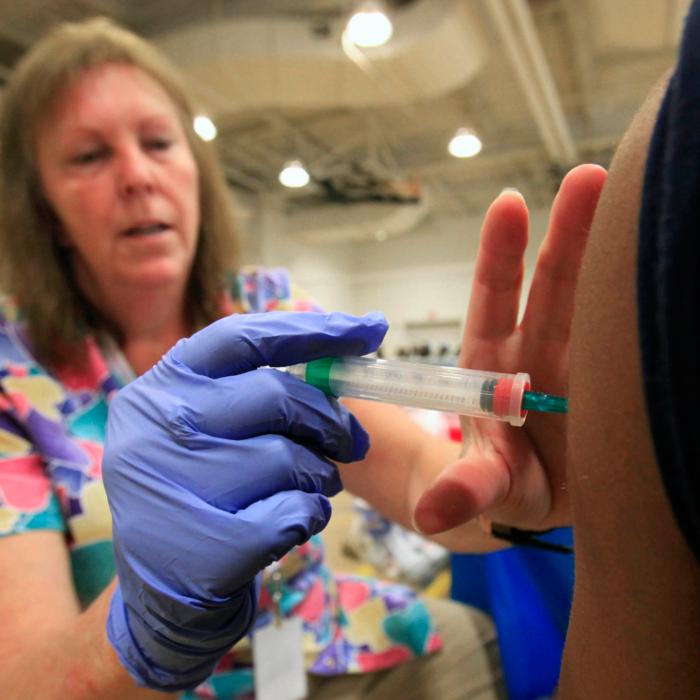
Older people and pregnant women are to be offered a new vaccine which the government says will protect against a usually mild respiratory virus that can occasionally cause serious complications in babies.
The announcement by the NHS comes as data show more women are choosing to have a vaccine-free pregnancy, amid increasing concerns about possible side-effects and efficacy.
From September, the over-75s and women who are at least 28 weeks pregnant will be offered one dose of the jab, which is said to protect against respiratory syncytial virus (RSV).
Made by Pfizer, the vaccine known as Abrysvo is part of the Medicines and Healthcare products Regulatory Agency’s (MHRA’s) Black Triangle Scheme, meaning it will be monitored more closely than established drugs for adverse reactions.
Premature Babies More at Risk
Babies—particularly if premature—are at more serious risk from RSV because their immune systems are not fully developed. The elderly, immunocompromised people, and those with heart and lung disease and are also more vulnerable to the illness.Data from the trial of the new jab “showed an excess of preterm births in the vaccinated group. A more detailed scrutiny of the Pfizer data by the committee showed no excess of preterm birth in HICs [high income countries], with the excess confined to the study sites in the upper middle income countries (UMIC),” according to the government.
Safety Signal for Premature Birth With Rival Vaccine
A rival RSV vaccine in development by GSK was withdrawn as a candidate in 2022 after a signal for premature birth was detected. Some experts criticised Pfizer for not informing pregnant women in its trial that GSK’s similar vaccine was halted over the pre-term birth risk, according to an investigation by the British Medical Journal.The RCPCH said RSV is responsible for around 33,000 hospital admissions of under-5s and between 20 and 30 deaths of young children every year in the UK.
NHS England aims to vaccinate as many people as possible in September and October before winter starts and seasonal illnesses, including RSV, circulate more widely. In Scotland, the jab will be rolled out from August, with Northern Ireland and Wales expected to follow suit.
A so-called “catch-up” campaign for those aged 75 to 79 will also be launched, with pharmacies and doctors’ surgeries expected to deliver the jabs to those who want them.
Dr. Conall Watson, consultant epidemiologist at the UK Health Security Agency, said: “RSV is a common respiratory virus that that can cause serious lung infections, like bronchiolitis and pneumonia.
“The vaccine will help prevent older adults and small babies from developing more serious complications from the virus whilst helping to reduce pressure on NHS services during the busy winter months.”
The new programmes follow guidance from the JCVI, which advises the government on jabs and considers the “economic burden” to the NHS as part of its decision-making process.
Dr. Mike McKean from the RCPCH said: “Campaigning for an RSV vaccine has been a key issue for the college for many years now and today’s announcement represents a huge step forward for child health and the paediatric workforce in England and Scotland.
“This newly announced programme has the potential to transform child health services during the winter months by reducing hospital admissions and could even save young lives.”
A vaccine against RSV has been sought for decades by the pharmaceutical industry.