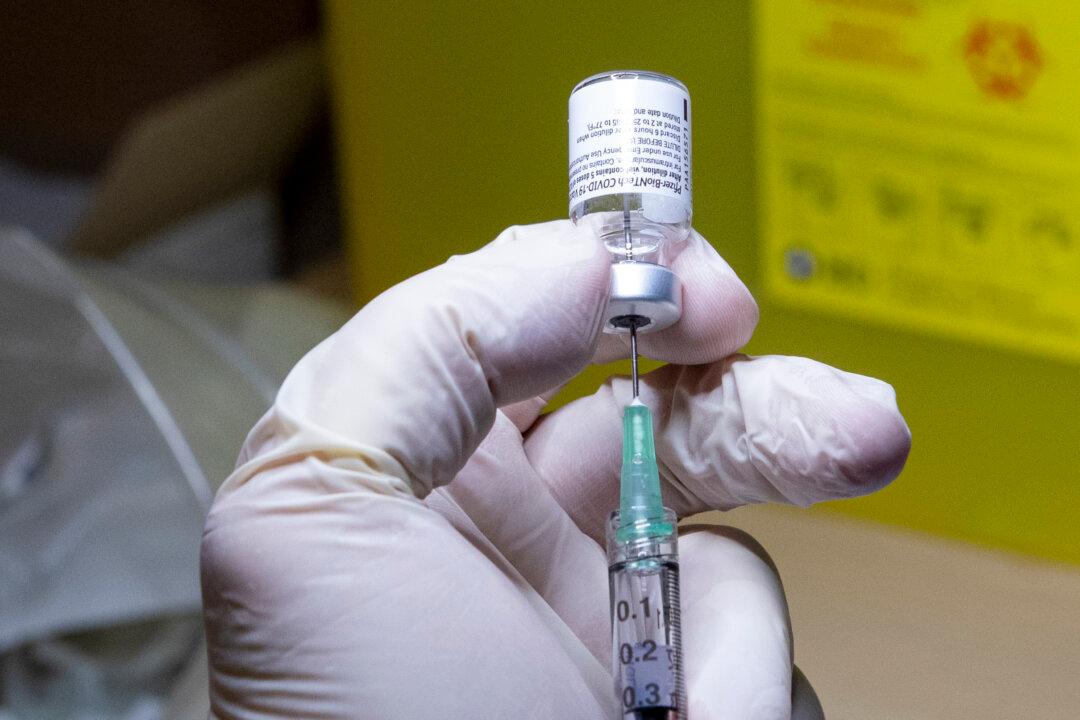
Pfizer and BioNTech say they will ship fewer COVID-19 vaccine vials to Canada if Health Canada agrees to change the vaccine label from five to six doses.
In December 2020, hospital pharmacists in the United States discovered that the vaccine glass vials that supposedly can only hold five doses, allows for a sixth if they use smaller syringes or special ones that trap less vaccine around the needle after an injection.