Canada is due to receive the first shipment of ID NOW COVID-19 tests this week and the quicker Panbio rapid tests by the end of the year,.
Health Canada approved a purchase of 7.9 million ID NOW rapid tests from Abbott at the end of September.
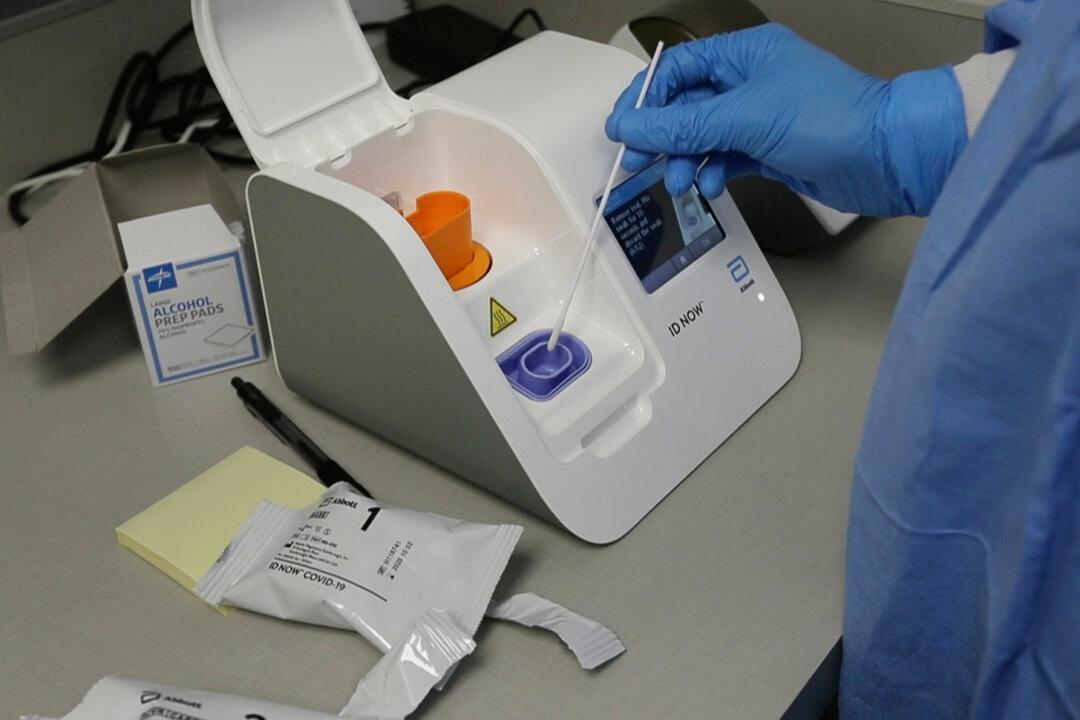
Canada is due to receive the first shipment of ID NOW COVID-19 tests this week and the quicker Panbio rapid tests by the end of the year,.
Health Canada approved a purchase of 7.9 million ID NOW rapid tests from Abbott at the end of September.