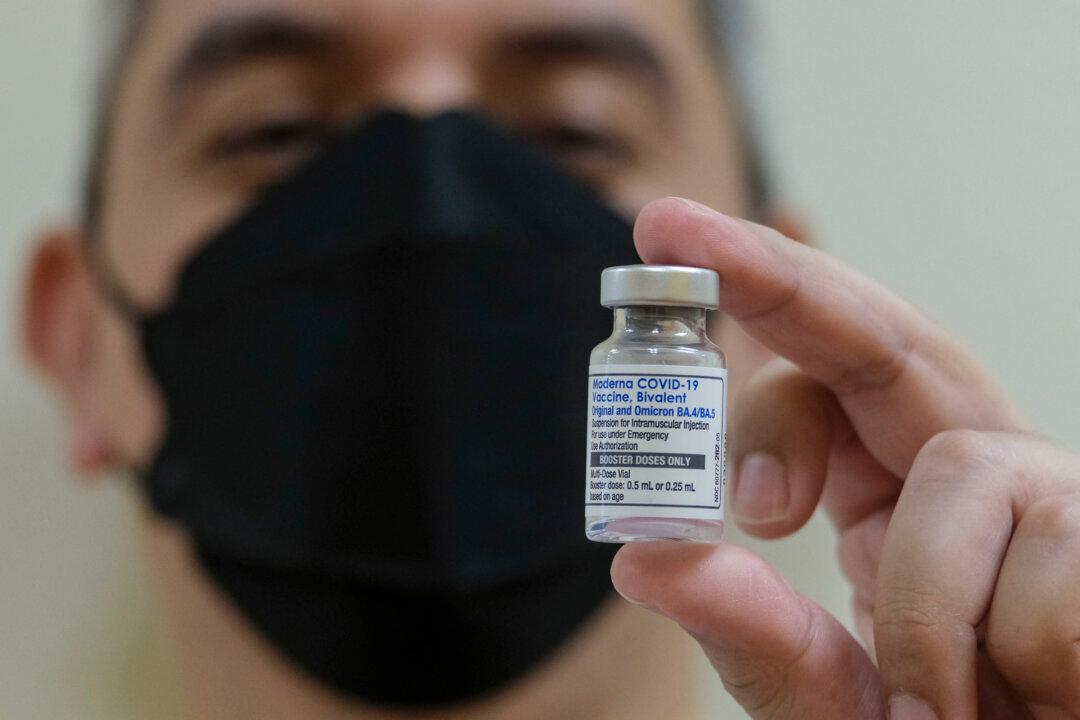
Health Canada has approved a new Moderna COVID-19 vaccine for all Canadians over the age of six months based on preliminary clinical data.
Health Canada posted authorization of the new shot on Sept. 12, saying the vaccine targets an Omicron subvariant called XBB.1.5.