An ex-big pharma supply chains consultant has told of his disbelief at the speed with which the COVID-19 jabs were brought to market and the dangerous way the manufacturing was carried out.
Mr. Rees, who has long called for reform of the pharmaceutical industry, said he was “astounded” at the speed of approval for the vaccines, with the rollout beginning just over nine months after the World Health Organisation declared a pandemic in March 2020.
The former engineer, who graduated from Cardiff University before joining the pharmaceutical industry in 1980, said that most people do not give any thought to the manufacturing process behind the drugs they are prescribed by doctors.
“In the aviation industry, with the standards they have, you wouldn’t give a part-finished aircraft to someone in an airport and say, right, you put the wheels on and do this little tweak to the wings, and then you’re away. We’re not going to tell you how to do it, we are going to give you some rough instructions. But there’s no standard operation procedures, no quality system. And that’s what’s happened in the vaccination centres.”
Temperature Sensitivities Could Make Jabs ‘Non-Sterile’
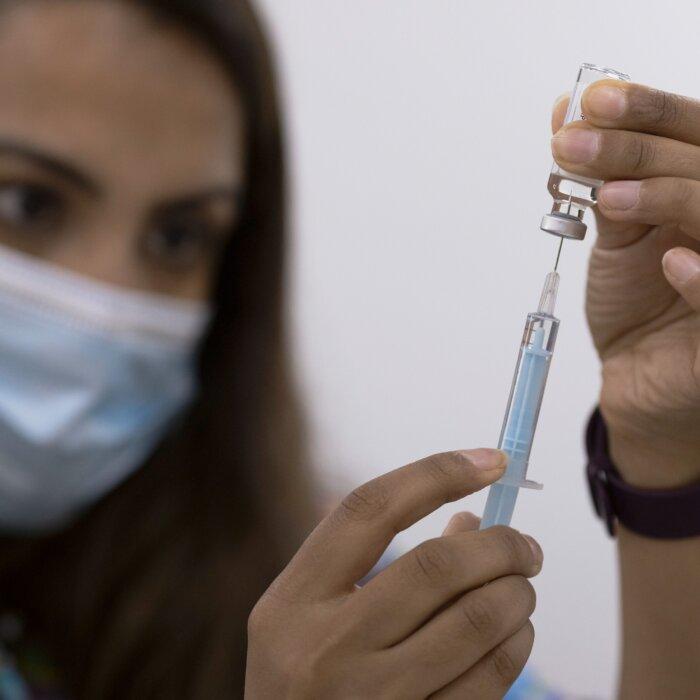
Mr. Rees added that the various biological components were all temperature sensitive and if any were stored incorrectly, this could render the vaccines “non-sterile” and “potentially highly potent.”“People have to thaw them, which is a manufacturing operation, it’s crucial [that] it has to be done under controlled conditions.”
“You have to study the profile of the thawing to make sure that what is left thawed and in the refrigerator is the same molecular composition as when it was frozen.”
He said that the jab rollout was the first time in the history of vaccination that the end stage of the manufacturing process was carried out by people outside of the industry without pharmaceutical expertise, as previously all vaccines arrived at a doctor’s surgery or hospital in single doses.
But with the unprecedented desire to vaccinate the whole world at high speed, Mr. Rees said that an untrained person was required to inject a saline diluent salt solution into the vials containing the doses and then turn them over ten times to mix it up again.
‘Hot Spots’ of Vaccine Damage
In his opinion, a non-expert volunteer incorrectly mixing the vials could explain the apparent “hot spots” whereby clusters of people who were given jabs from the same batch suffered serious harm—including death—whereas many others appear to have suffered no ill effects from the vaccines.“People in vaccination centres doing that, you know, they could have been the hotspot in one part. And that’s one of the theories that I’ve come to [as to] why some people had bad—very bad—reactions and others nothing at all.”
“Any stage in the manufacturing process can make a drug toxic,” he said. “We know that supply chain adulteration as they call it, has killed people, because toxic materials have been substituted for the real thing. And in 2011, there was a falsified medicines directive that was passed in Europe to make sure that that couldn’t happen again.”
Defenders of the vaccines—including the government, which continues to insist they saved “millions” of lives—say that unprecedented resourcing was out into the clinical trials phase, leading to the incredibly fast development of the drugs. But Mr. Rees said that speeding up the manufacturing supply chains cannot be safely done.
“Because they focus on the clinical element—which you can set up a clinical trial, you recruit patients, you can do it in months—whereas you can’t build a supply chain in months. It takes three years to build a preclinical supply chain before you can go into humans.”
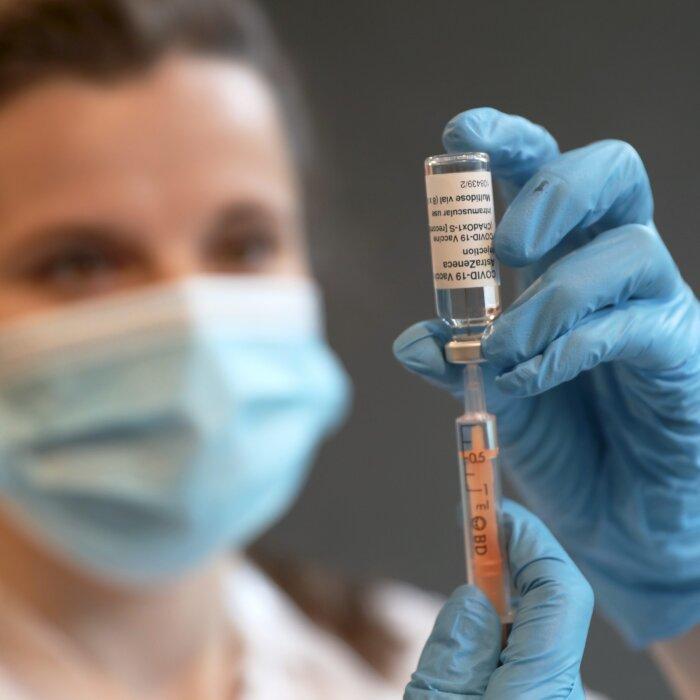
He expressed further astonishment at the inspection process by the Medicines and Healthcare products Regulatory Agency (MHRA) after Oxford Biomedics was allowed to take over a former post office in Oxford to manufacture the AstraZeneca vaccines.
“That was approved by the MHRA in months, which is absolutely unheard of ... and you have to wonder why. Because that was all dangerous, you know, new equipment you have to validate, you have to prove that what you say you’re gonna make on the new equipment actually is what you made.”
Focus Was on ‘Creating Blockbuster’ Drugs
Speaking about his four decades in the industry, Mr. Rees said that standards had steadily declined since he joined, with more and more outsourcing of the manufacturing process and many sub-contractors and consultants involved.“The whole of Big Pharma is now locked in to supply chain that they’ve got no control over in terms of cost, or in terms of the location or inventory in terms of who’s doing what. Everyone knew that the standards in the industry were poor because the focus was on getting drugs to market, creating bulk blockbusters, and returning mega profits to invest in.”
He adds that the UK has always been a “moving force in pharmaceuticals,” with companies such as GlaxoSmithKline and AstraZeneca and that the UK played a major role in bringing the new vaccines to market, working in conjunction with billionaire Bill Gates, who he says appears to be “building a global supply chain.”
“Mr. Gates has been working with Britain in terms of you know, putting money into the MHRA, talking to government ministers ... and it looks to me that the MHRA and Britain has become the central global hub for the rollout of these injections, because the MHRA was first to approve the Pfizer vaccine.”