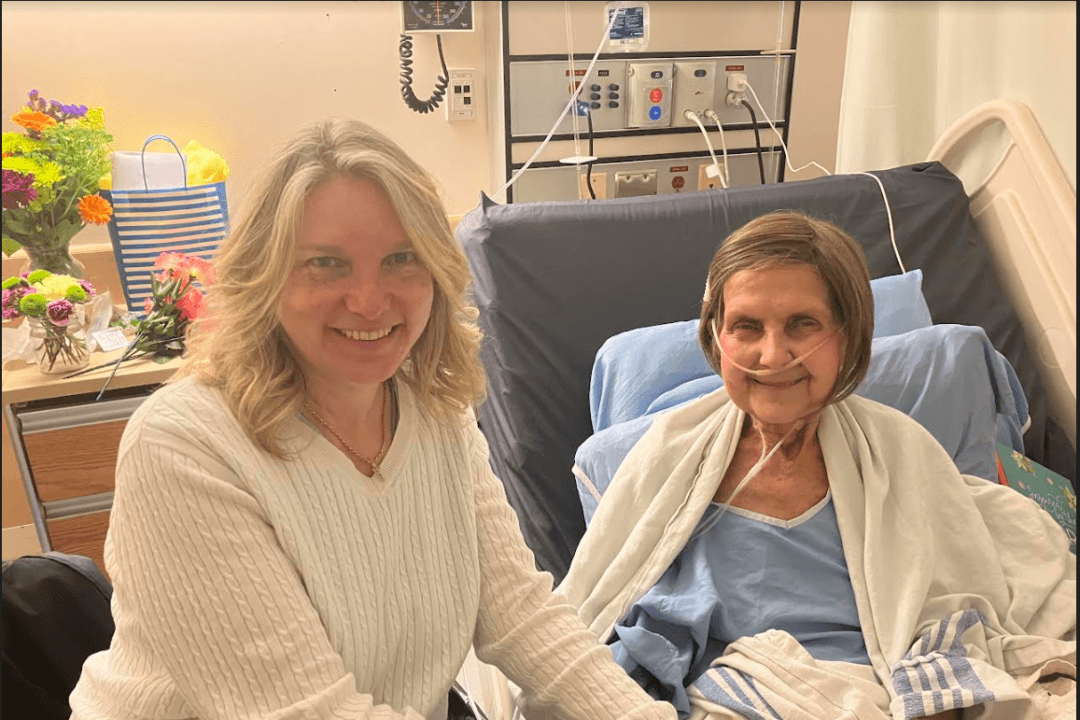
Ross Wightman, who was diagnosed with Guillain-Barré syndrome shortly after receiving a dose of the AstraZeneca COVID-19 vaccine, has become one of the first recipients of funds under Canada’s Vaccine Injury Support Program.
But the plight of others who have suffered vaccine injuries weighs heavily on the realtor and former pilot from B.C.’s Okanagan Valley.