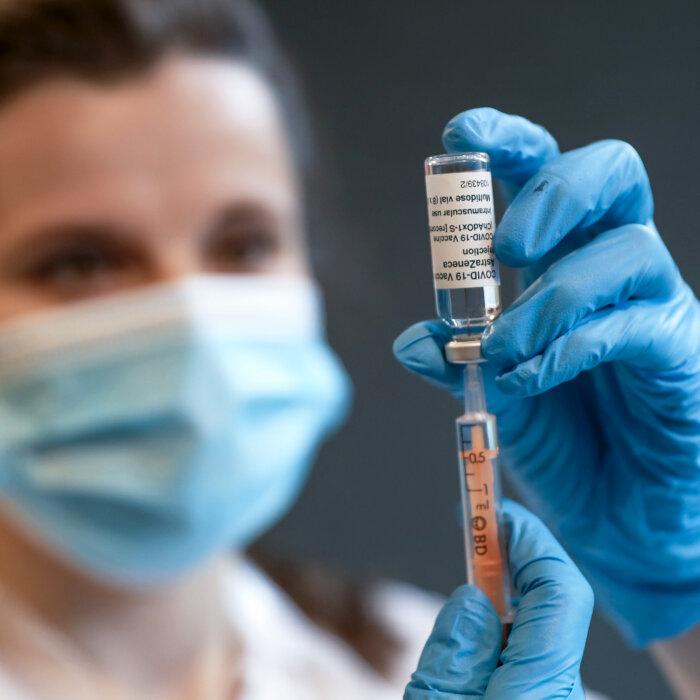
Participants and experts involved in the UK Covid-19 Inquiry have claimed that important lines of inquiry regarding the manufacturing process were not pursued in the module looking at vaccines and therapeutics.
The three weeks of evidence given over to Module 4 of the inquiry concluded on Jan. 31 after hearing from representatives of the vaccine-injured and bereaved groups as well as from officials responsible for the jab rollout.
Ruth O'Rafferty, who gave evidence on behalf of the Scottish Vaccine Injury Group, told The Epoch Times she believes this portion of the inquiry focused on the need to reform the Vaccine Damage Payment Scheme (VDPS) and the Yellow Card reporting system for adverse reactions, rather than looking at why the jabs were approved in the first place.
‘Silenced’
Reflecting on her experience giving evidence on Jan. 15, O'Rafferty said: “I was absolutely silenced. I was sat in the side room with [lead counsel to the inquiry] Hugo Keith telling me what I was allowed to say before I went in.”She said she was told she was “way out of her scope” in her statement because she was referencing scientific studies, and as she was not an expert witness, these matters would not be touched upon when she was questioned.
“I was disgusted at the fact they weren’t looking at vaccine safety in any great depth,” she said, pointing out the length of time given over to this segment of the module had been reduced from five to three weeks, which she believes was “terribly short” for such an important subject.
Process 1, Process 2
Dame June Raine, head of the Medicines and Healthcare products Regulatory Agency (MHRA), appeared before the inquiry on Jan. 22, where she was questioned on the matter of the manufacturing switch that O'Rafferty had tried to raise.Keith said: “Some have suggested that the batches which were delivered to the United Kingdom for use amongst its population, which were then handed out, were not the same batches, or rather were batches that were produced by a different manufacturing process on the part of the manufacturer, as had been tested by the MHRA.
“So bluntly, the suggestion has been made, you tested and authorised and certified a certain number of vaccines made by process, manufacturing process A, and then the manufacturers actually delivered vaccines to the British population produced as a result of a different manufacturing process, and one, by inference, which had not been tested. Is that right?”

Raine replied, “Well, my understanding is that the manufacturing process would have been the same.”
The vaccines made by Process 2 were tested on just 252 people before being approved by the MHRA, with the results of this small trial never made public.
Questioned about the testing of the vaccine batches, Raine said that the official medicines control laboratories run by the MHRA “test them for purity and potency, so that every person who has a vaccine will get one that works and doesn’t have impurities.”
Raine said that as there could be more than a million doses in each batch, it wouldn’t be “appropriate” to test each one, but that a “relevant” number of vials would be tested, and “rigorously assured for purity and potency.”

‘Bait and Switch’
A former consultant to the pharmaceutical industry with 40 years of experience specialising in supply chains, Hedley Rees, told The Epoch Times that Raine “must” understand that any change in the manufacturing could alter the end product, because with temperature-sensitive biologics, “the process is the product.”He said, “This is what [former MP] Andrew Bridgen was calling bait and switch ... If you change anything about the process at all, you have to test it again. You have to put it through animal model tests again, then human, because when you change the process, it could change the molecular structure of the product, and it could become a different product.”
Rees writes his whistleblowing blog, Inside Pharma, and says he has “given up” with official processes like the inquiry as a way of holding anyone to account for what he regards as “the greatest crime against humanity in the history of the world.”
“The trouble is the lawyers don’t know what they’re talking about, so they don’t ask the right questions,” he said, adding that the AstraZeneca jab was “effectively” the same as the Pfizer and Moderna ones, although it used the viral-vector technology instead of mRNA.

Speaking to The Epoch Times, Craig said she believes it is possible for the barristers to understand the complexity of the science behind the vaccines, and that questions being asked of expert witnesses are a deliberate choice.
“I think there’s nothing about medicine which is not accessible to people who put the time in ... It’s not some kind of sacred knowledge. It’s just knowledge.
‘Deliberate Muddling’
Craig, a co-founder of The People’s Vaccine Inquiry, said the question that Keith ought to have asked Raine was, “Did the MHRA have [trial] data for humans for Process 2 or only Process 1?”In her view, Keith was “deliberately muddling” by referring to Process A, and “asking a similar question on testing the batches to avoid asking the actual question.”
“I think they fed her a question,” she said.
“What [Keith] really asked was, are the batches the MHRA tested the same as the ones that went into the public? And they are—because the MHRA tested Process 2 batches,” she said, adding that it had been difficult to pin the regulator down on what tests had been carried out on the batches.
“The things that they have in the public domain have been about testing RNA quantity and quality, nothing else,” she said, adding that this relates to “efficacy” rather than safety, such as looking for contaminants that have been found in some batches.

“Manufacturing steps that were not scalable were replaced with those designed to provide a similar or better impurity profile. This ‘Process 2’ drug substance was required to be shown comparable through side-by-side comparability studies and heightened characterisation testing. New processes were validated at all manufacturing sites and submitted for review and approval. Vaccines produced by both ‘Process 1’ and ‘Process 2’ were included in the clinical trials.”
A spokesperson for the UK Covid-19 Inquiry told The Epoch Times in a statement that it “completely refutes” the suggestion that Keith was “deliberately muddling” his words in his questioning of Raine.
“All witnesses gave evidence in public. Their testimony can be viewed on our YouTube channel and transcripts and other associated evidence was published on a daily basis throughout the hearings,” the statement added.
“In addition to evidence provided during hearings, the inquiry will be considering substantial quantities of written evidence, which will be published shortly. The inquiry’s chair, Baroness Heather Hallett, has not yet reached any conclusions as to any of this evidence.
“No witness is asked to sign a non-disclosure agreement as a condition of giving evidence.
“UK Covid-19 Inquiry processes relating to the confidential handling of evidence are standard processes adopted at other UK public inquiries. Core Participants (CPs) are required to sign a confidentiality undertaking which stipulates that any documents provided to them in their role as a CP must be kept confidential until such time as they are made public.”
Hallett’s Module 4 report, setting out her conclusions and recommendations, is expected next year, while Module 5, examining contract procurement, will begin in March.