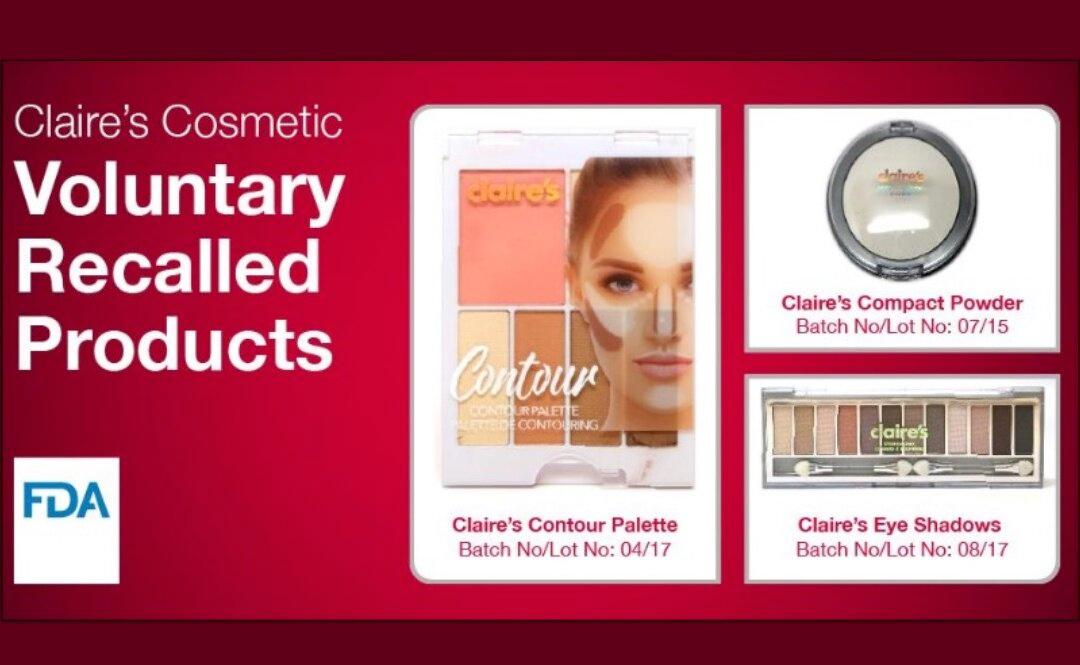
Health Canada issued a recall for three of Claire’s cosmetic products after testing showed possible asbestos contamination, following the same recall in the United States last month.
Health Canada issued the recall on April 2 after tests indicated the possible presence of asbestos fibres in product samples from one lot each of Claire’s contour palette, Claire’s eyeshadows, and Claire’s compact powder.