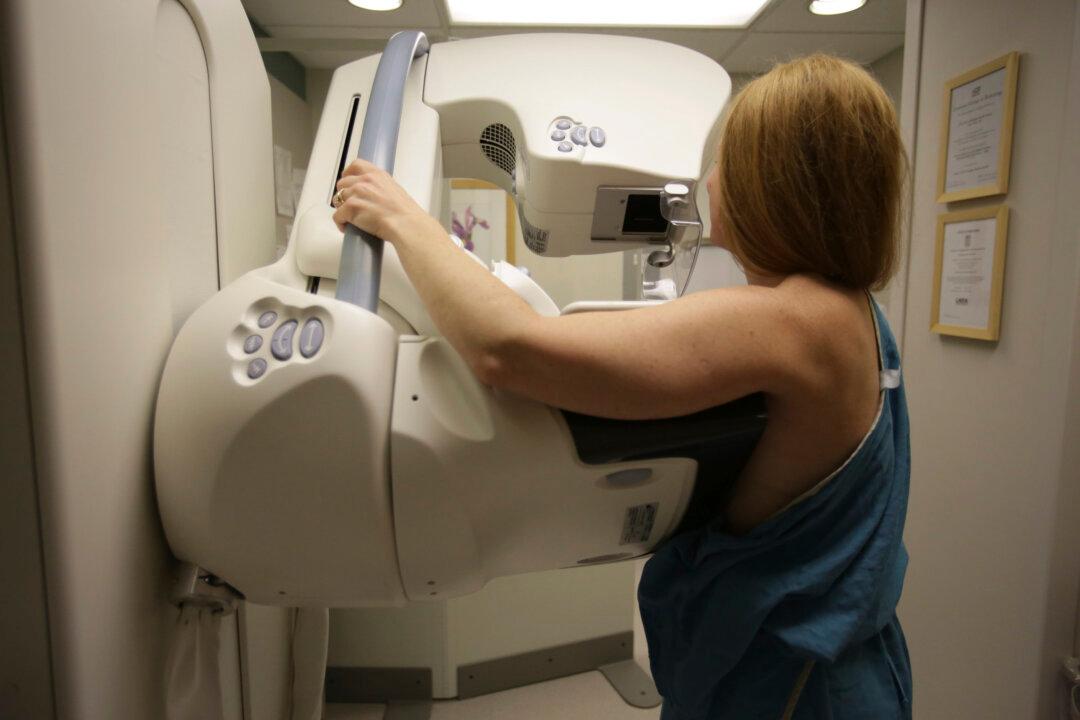
Thousands of Canadian women in their 40s may have died needlessly from breast cancer due to a lack of access to mammographies as a result of two “skewed” studies that informed guidelines recommending against screening for this age group, a team of researchers says.
In a commentary published in the Journal of Medical Screening on Nov. 23, researchers from five universities said they have new evidence that the Canadian National Breast Screening Study trials conducted in the 1980s were not randomized properly, leading to unreliable results.