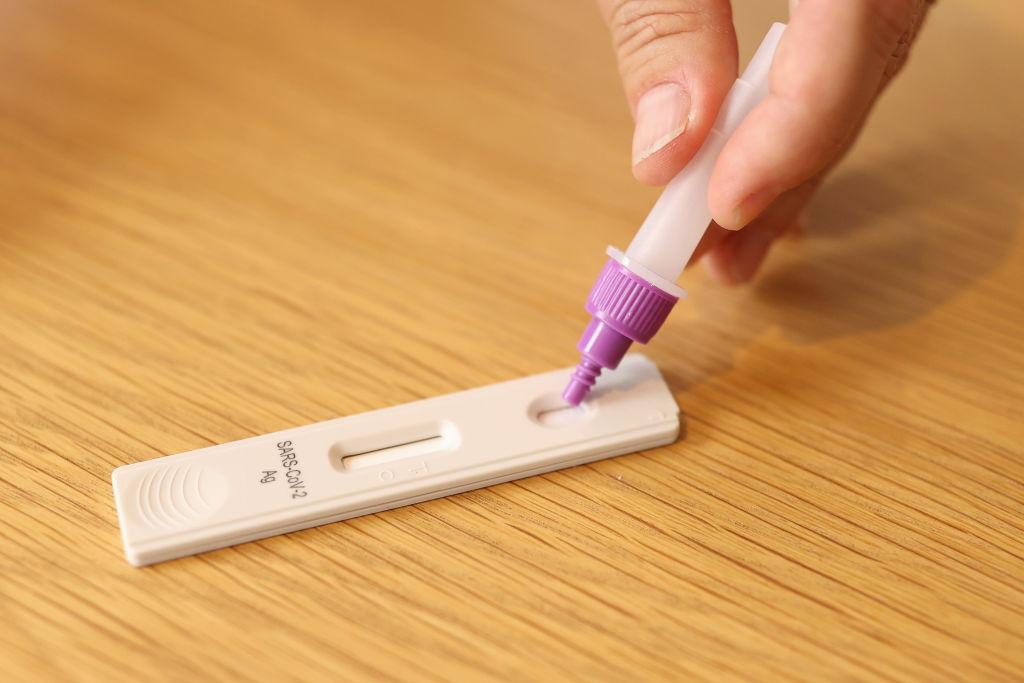
Vulnerable Australians will be able to access COVID-19 rapid antigen tests far more easily after the release of a new one-step saliva rapid antigen test.
The test which is the brainchild of the Australian-based digital health company Gardian took 12 months in the making and is a “non-invasive, saliva-based rapid antigen test that is suitable for all ages and abilities including those with low-vision, are elderly, have issues with physical dexterity or have cognitive disabilities.”