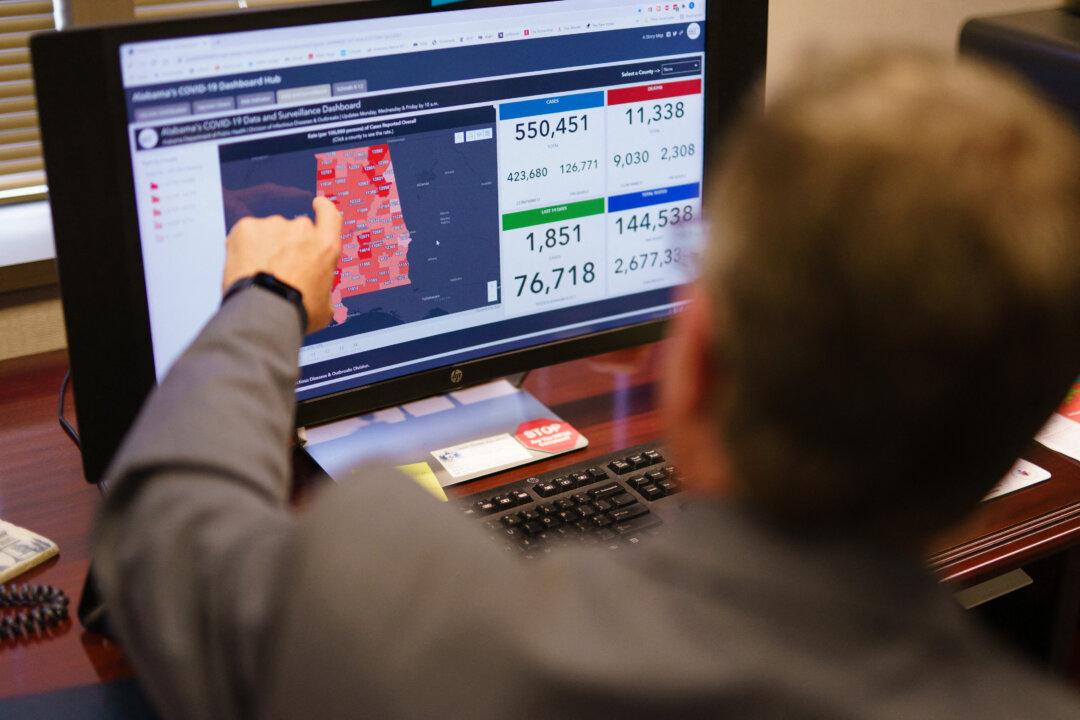
The federal government’s sudden rationing of monoclonal antibody treatments, which are keeping Americans who get COVID-19 out of hospitals, is hitting Alabama hard, with some sites already running out of or projected to run out of supply soon.
The antibodies are highly successful at stemming the effects of COVID-19, which is caused by the CCP (Chinese Communist Party) virus, when given to patients soon after they contract the disease. But a huge jump in demand in recent weeks has left what some officials have described as a national shortage, triggering the federal government to intervene and start doling out what’s left.