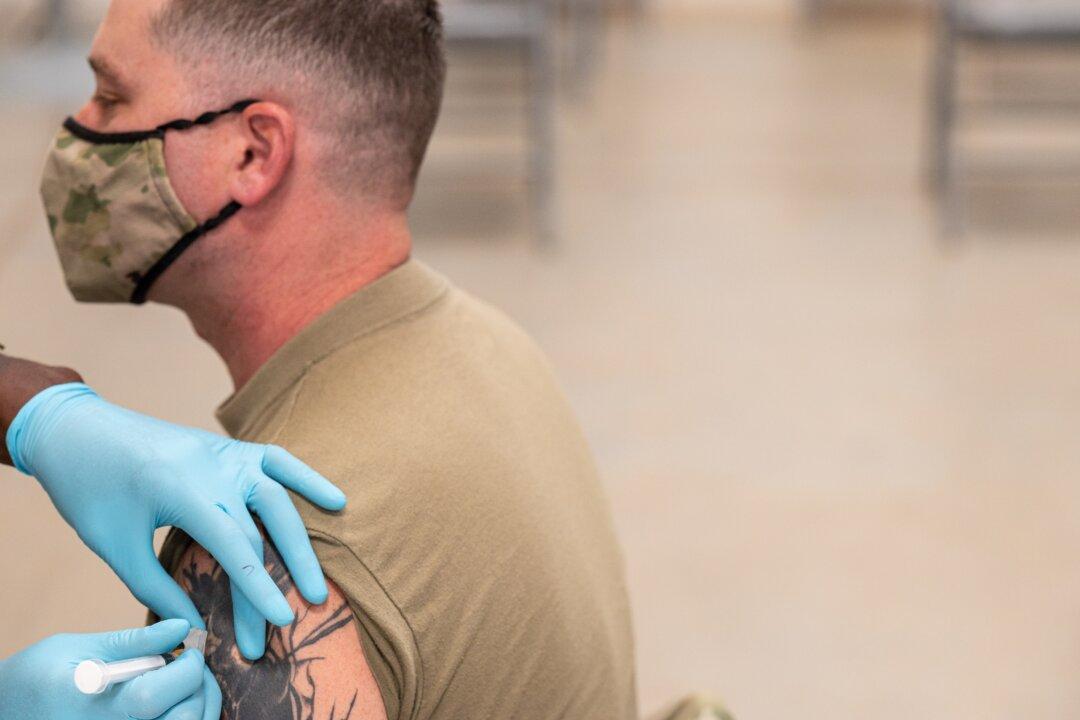
Scientists at the Walter Reed Army Institute of Research (WRAIR) say they have developed a vaccine that is effective against all the variants of the virus that causes COVID-19, including Omicron and other strains that may not yet exist.
A series of preclinical study results had shown that the vaccine they have developed, known as the Spike Ferritin Nanoparticle (SpFN) COVID-19 vaccine, “not only elicits a potent immune response but may also provide broad protection against SARS-CoV-2 variants of concern as well as other coronaviruses,” researchers in WRAIR’s Emerging Infectious Diseases Branch (EIDB) said in a Dec. 16 statement.