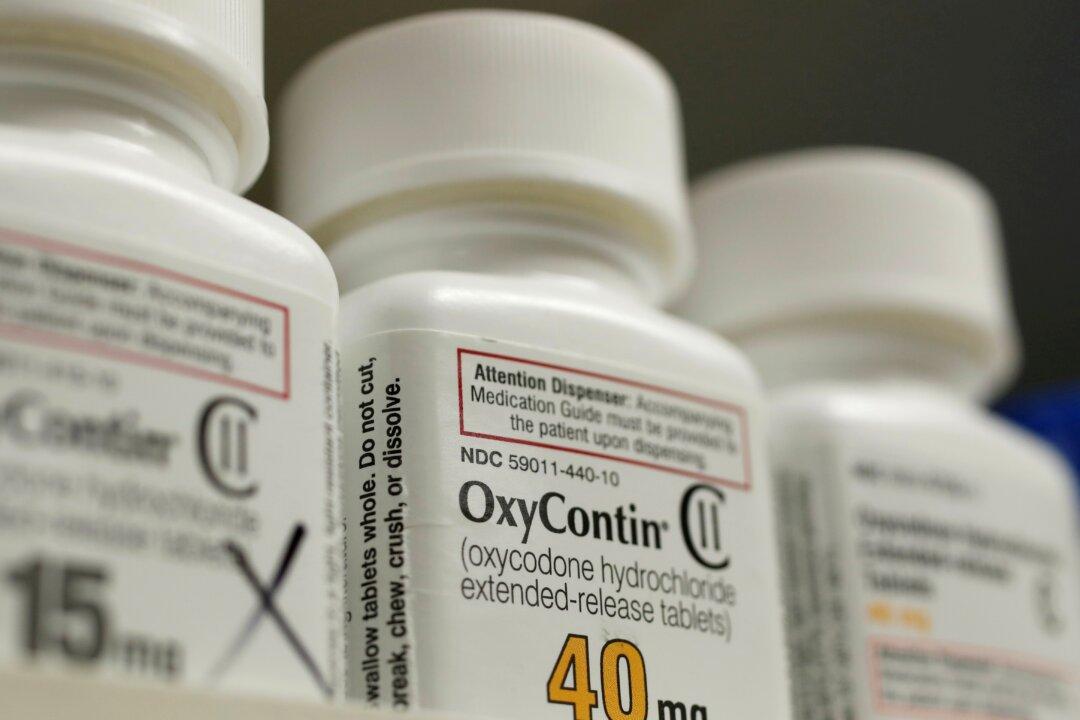
Consulting firm McKinsey & Company has reached a $650 million settlement with the U.S. Department of Justice (DOJ) for its role in helping boost the sales of OxyContin, a highly addictive opioid, for Purdue Pharma, according to court documents filed in Virginia on Friday.
The agreement allows the firm to avoid criminal prosecution if it pays the sum and follows specific conditions for five years, including stopping all work related to the sale, marketing, or promotion of controlled drugs.