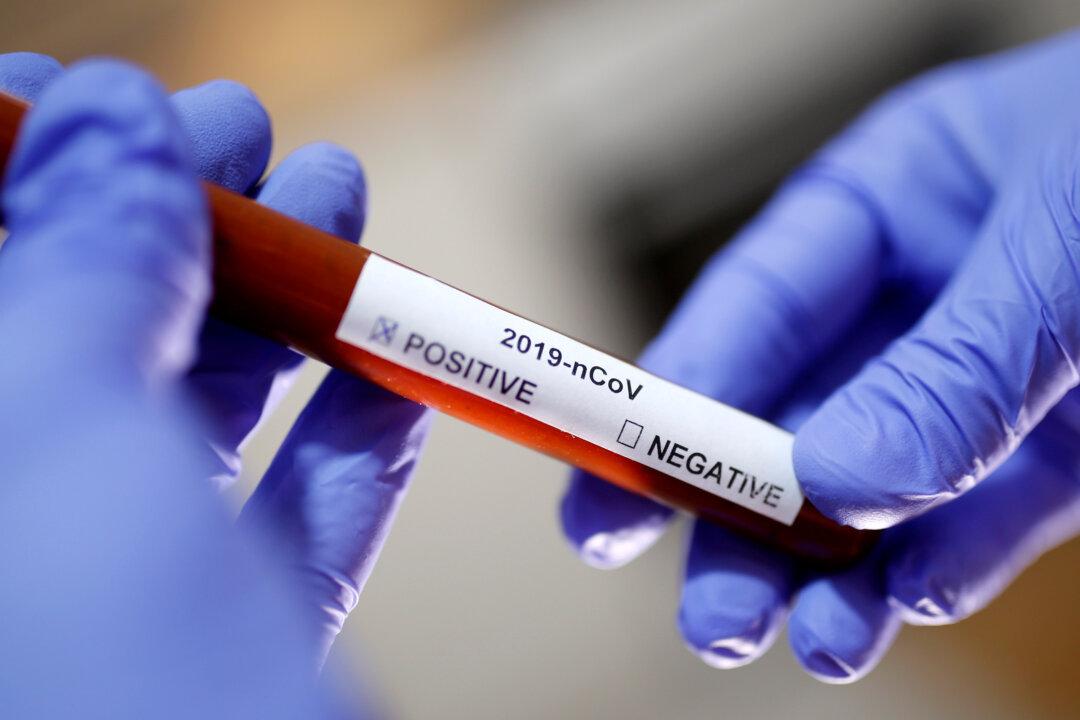
Public health laboratories in every American state are now able to test for the new virus.
Seventy-eight state and local labs have successfully verified testing kits manufactured by the Centers for Disease Control and Prevention (CDC) and are currently testing for COVID-19, Scott Becker, CEO of the Association of Public Health Laboratories, said on Monday.