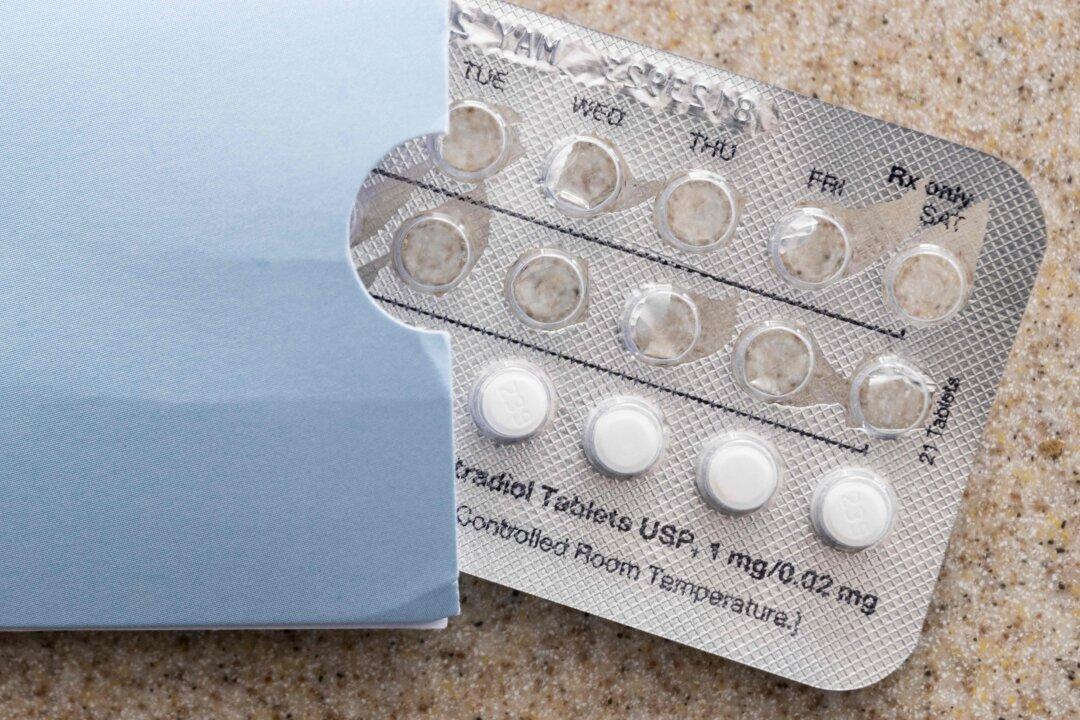
The U.S. Food and Drug Administration is warning consumers that two batches of the prescription birth control pill Tydemy may not be effective and could result in unexpected pregnancy.
The warning on Aug. 1 comes after Lupin Pharmaceuticals, the manufacturer of the Tydemy pills, issued a voluntary recall on July 29 over the same concerns.