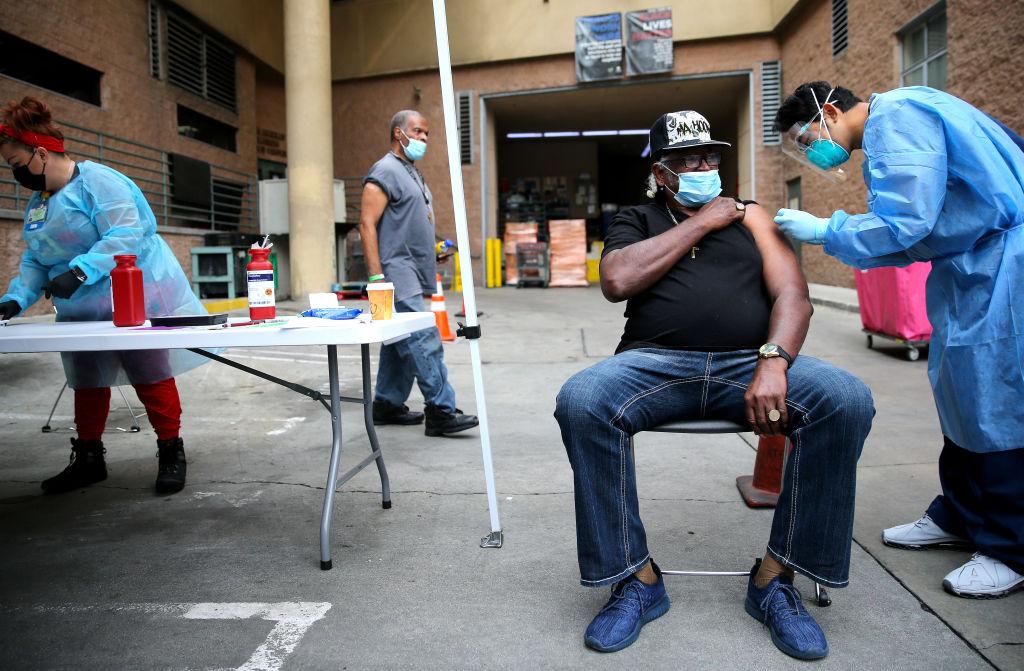
The Food and Drug Administration (FDA) stated on Feb. 24 that Johnson & Johnson’s single-dose vaccine prevents COVID-19 and that the FDA will make a final authorization decision soon.
Johnson & Johnson has an ongoing study of 44,000 patients in South Africa, the United States, and several other countries, with seven deaths recorded among patients receiving a placebo—and none among those who got the vaccine.