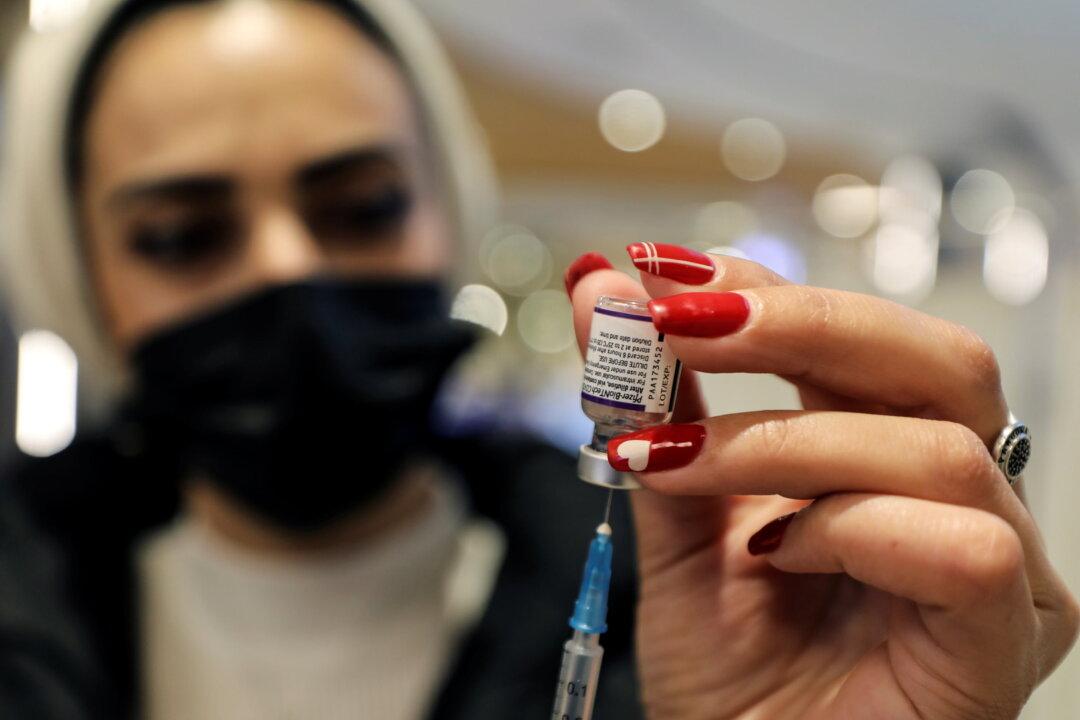
BEAUMONT, Texas—Attorneys for a whistleblower alleging violations occurred during the Pfizer–BioNTech COVID-19 vaccine clinical trial argued in federal court on March 1 that their case should advance to the discovery phase.
Attorneys for the defendants—Pfizer and two subcontractors, Ventavia Research Group and ICON—tried to convince the judge to dismiss the case. If successful, their clients would avoid depositions under oath.