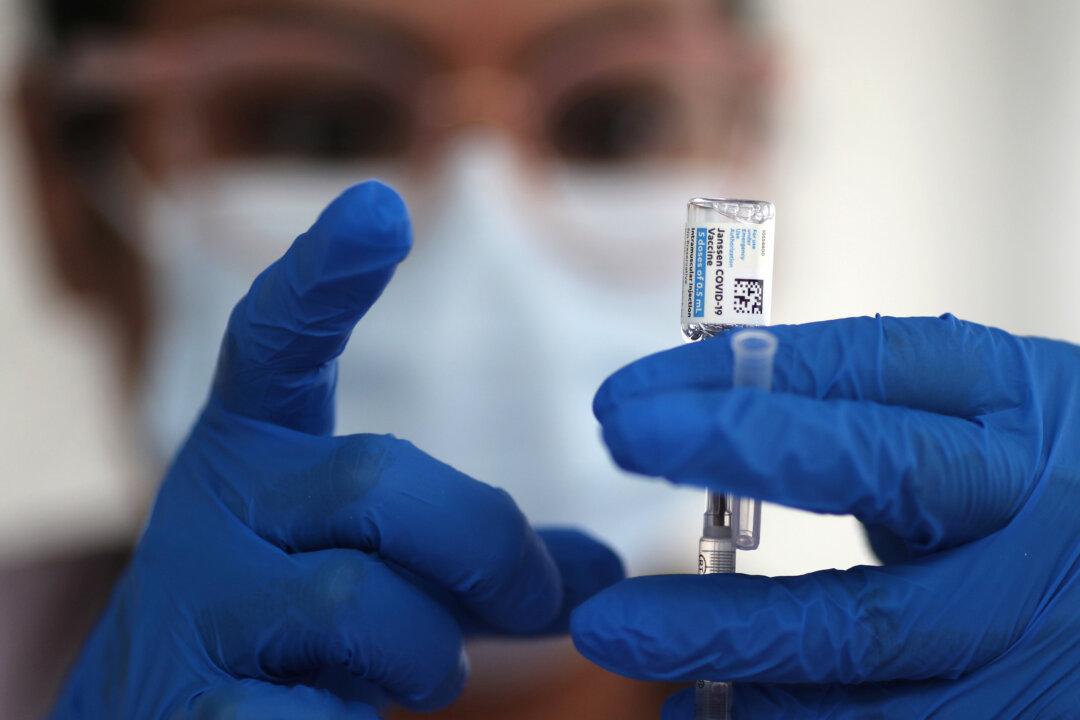
The Centers for Disease Control and Prevention (CDC) said on April 14 that it learned of a seventh woman who developed a rare and severe type of blood clot after receiving the Johnson & Johnson (J&J) COVID-19 vaccine.
The seventh possible patient was identified as a 28-year-old woman, as the Advisory Committee on Immunization Practices debates whether the J&J vaccine should be used. The committee said more information is needed to make an informed recommendation.