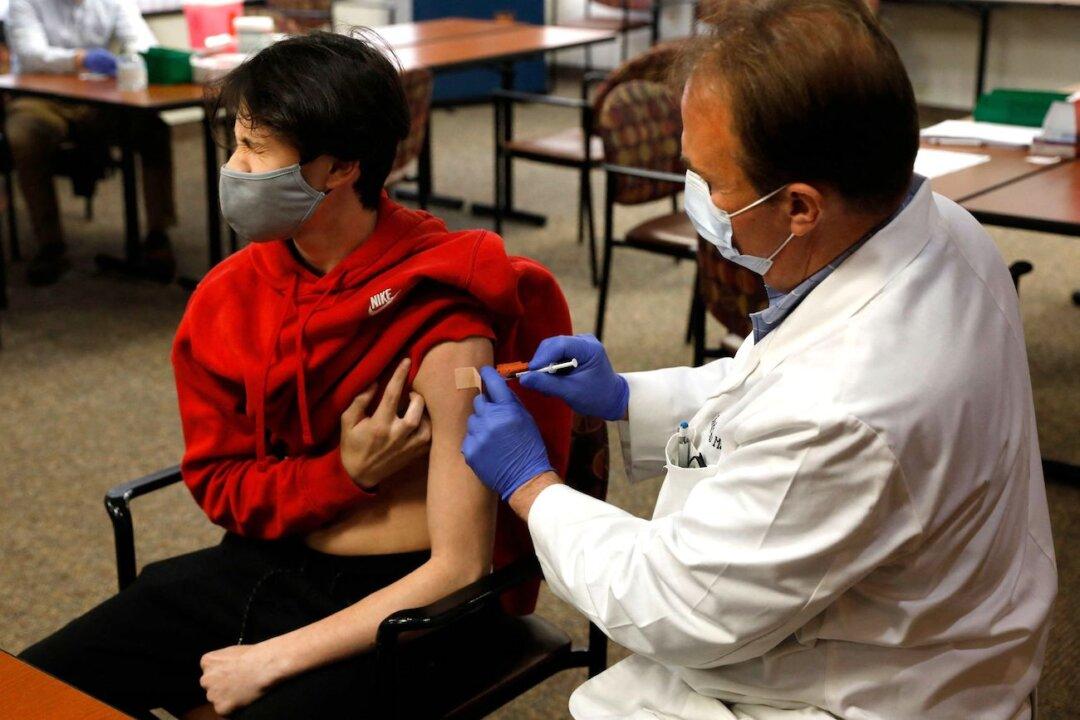
Pharmaceutical company Pfizer said it’s moving forward with trials of its COVID-19 vaccine for children between the ages of five and 11, and expects to make the vaccine available in the fall.
If studies show a good immune response and safety, it will then launch the vaccine for children aged six months to five.