Sandra Lindsay, a critical care nurse at Long Island Jewish Medical Center in New York, on Dec. 14 was the first American to receive a new COVID-19 vaccine.
“It didn’t feel any different from taking any other vaccine,” she said.
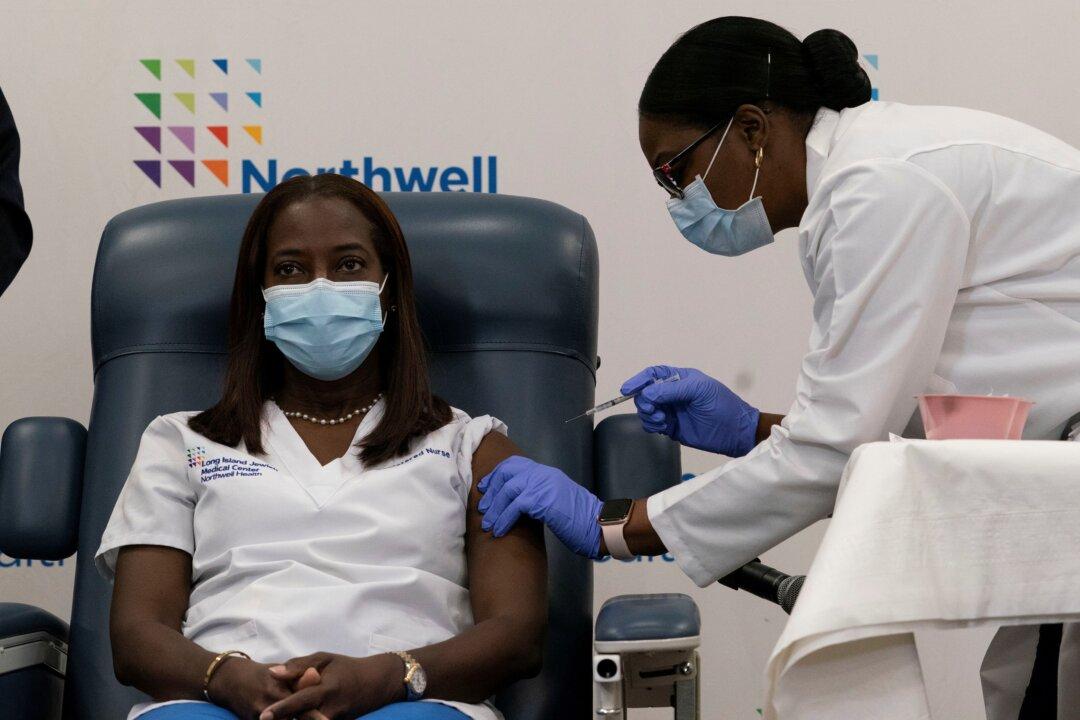

Sandra Lindsay, a critical care nurse at Long Island Jewish Medical Center in New York, on Dec. 14 was the first American to receive a new COVID-19 vaccine.
“It didn’t feel any different from taking any other vaccine,” she said.