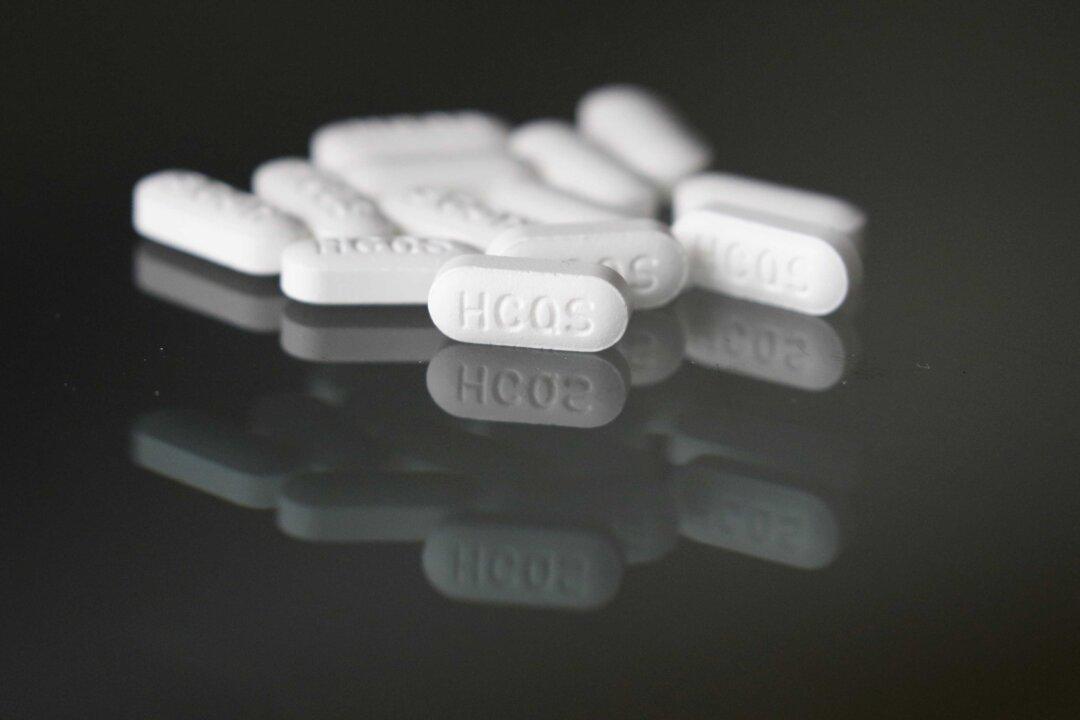
The National Institutes of Health (NIH) has halted a clinical trial to study the “safety and effectiveness” of hydroxychloroquine for treating the CCP virus in adults.
In a statement on June 20, the NIH said the “study shows treatment does no harm, but provides no benefit.”