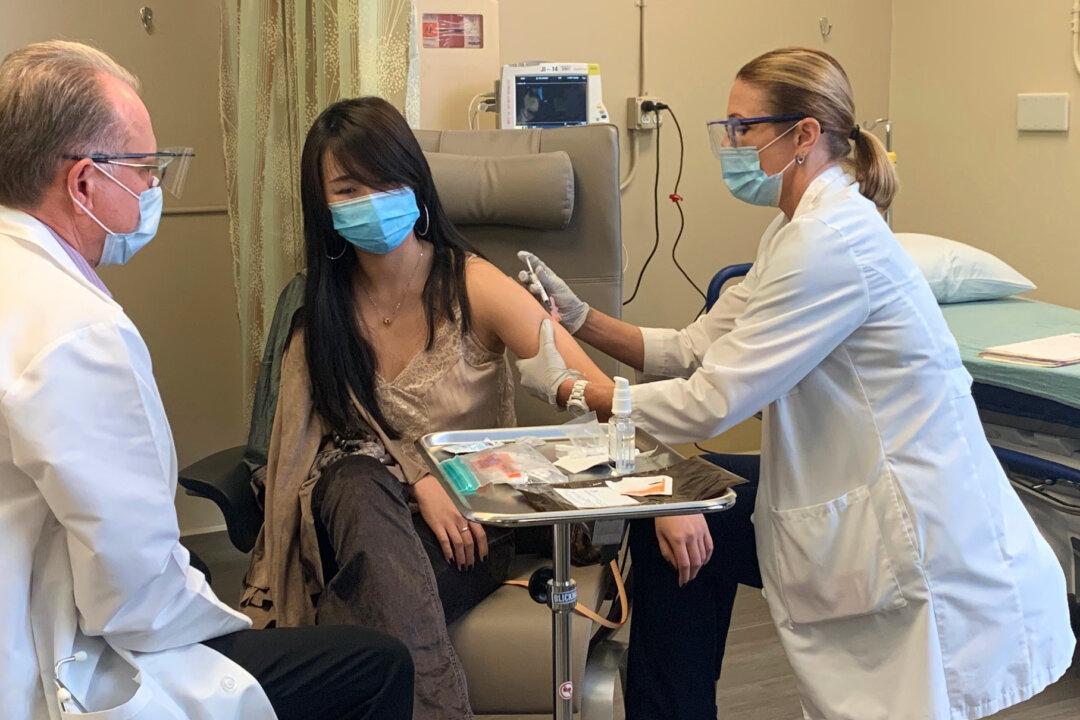
A 25-year-old recent graduate of the University of California–Irvine became the first person to receive a phase one dose of a new COVID-19 clinical vaccine trial at Hoag Memorial Hospital in Newport Beach, California.
Chen Cao became the first of 35 adults, ages 18 to 55, to be injected with the new trial vaccine when she received the shot on Oct. 21. After approximately three weeks, patients receiving the first shot of the new vaccine will return to the hospital to receive their second dose of the trial.