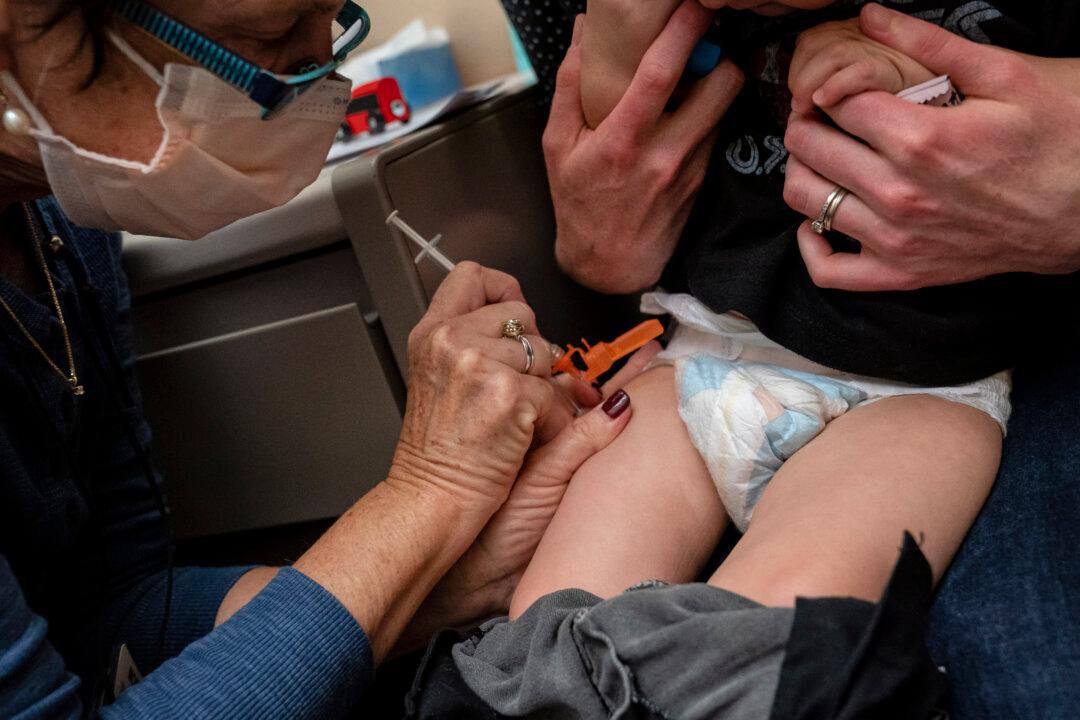
More than 100 young children suffered seizures after receiving a COVID-19 vaccine, according to a new study.
One hundred and four children under 6 years old suffered a seizure within 42 days of a COVID-19 shot, researchers with the U.S. Centers for Disease Control and Prevention (CDC) and other institutions found.