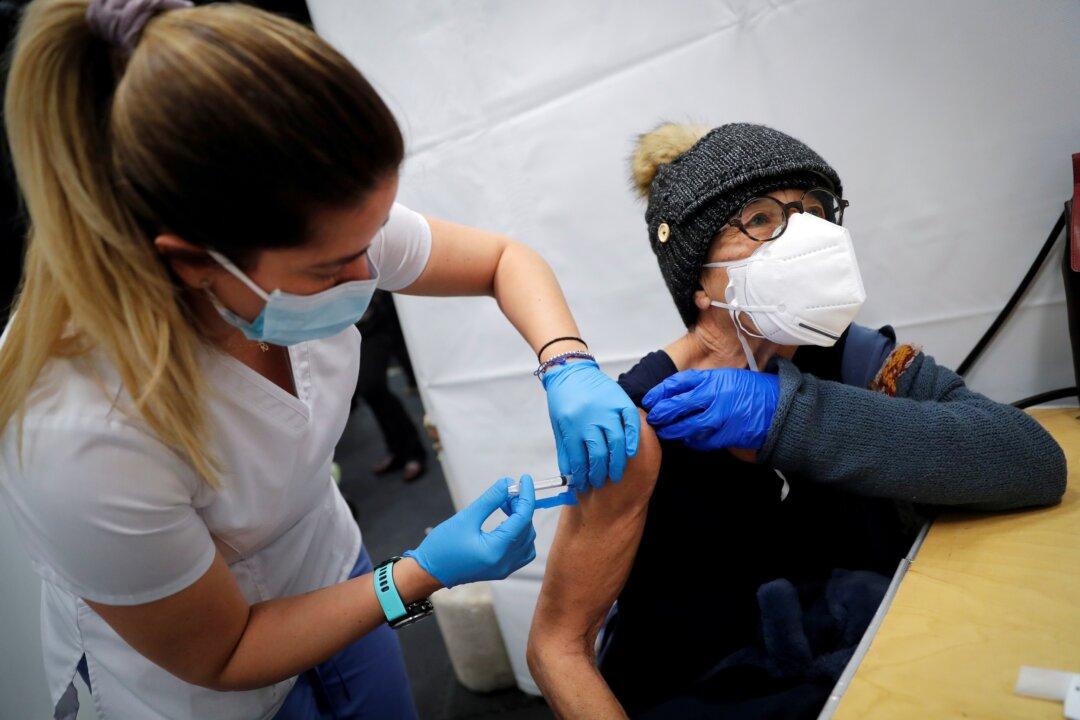
People who received the Moderna COVID-19 vaccine reported more side effects than those who got the Pfizer/BioNTech shot, according to a recent study by the Centers for Disease Control and Prevention (CDC).
The study—published online in the Journal of the American Medical Association (JAMA) on April 5—looked at data collected from over 3 million participants vaccinated from Dec. 14, 2020, through Feb. 28, 2021, in the CDC’s v-safe active surveillance system. However, only 1,920,872 participants reported getting the second vaccine dose.