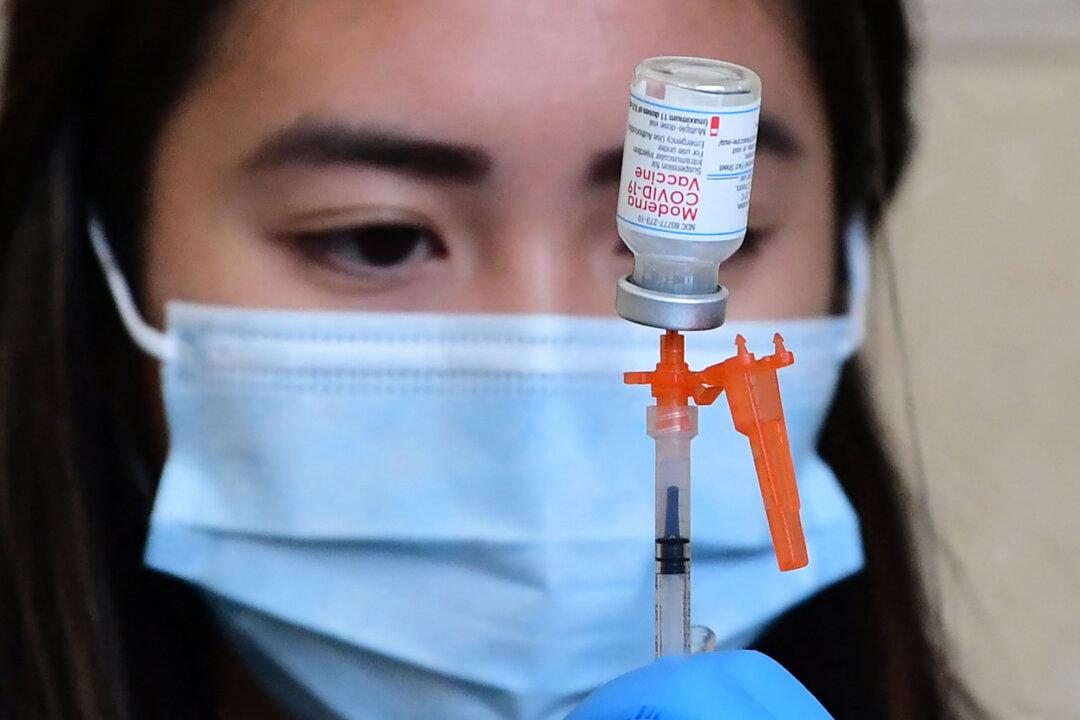
The first person has received Moderna’s COVID-19 vaccine booster that targets the Omicron virus variant, the company announced Wednesday.
The Phase 2 study is expected to enroll approximately 600 adults 18 or older, half of whom have received two doses of Moderna’s original vaccine and half of whom have received both the primary two-dose series and a booster that doesn’t target Omicron.