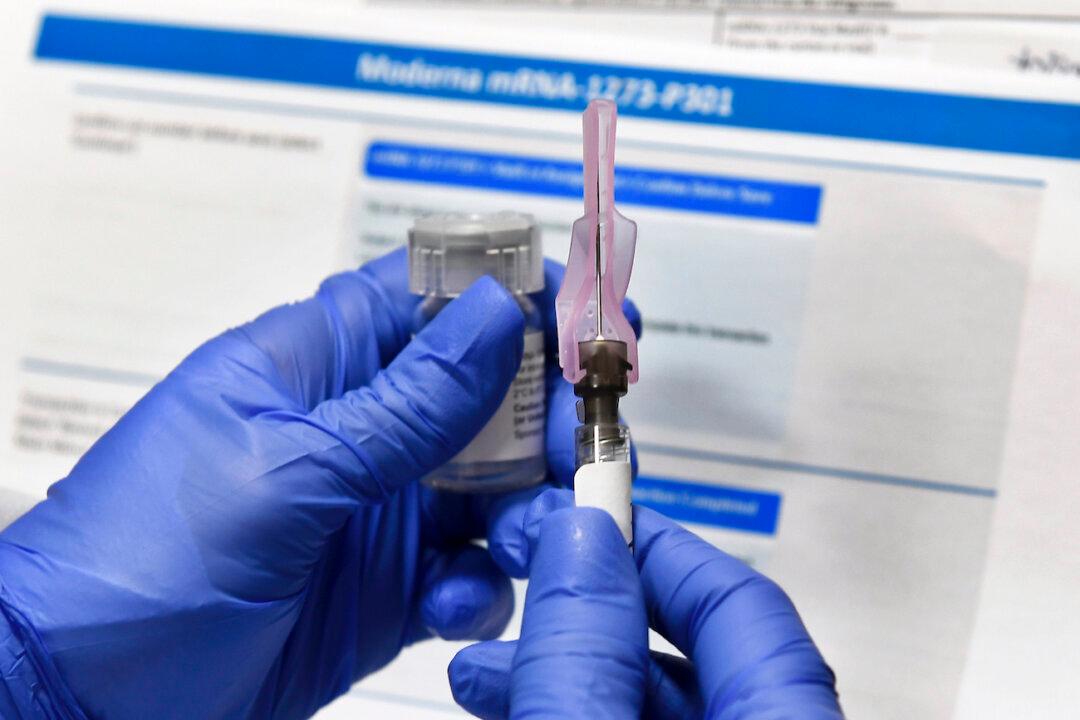
A panel of advisers to the Food and Drug Administration (FDA) voted on Thursday to endorse for emergency use a COVID-19 vaccine from Moderna and the National Institutes of Health.
The advisers voted 20-0 with one abstention, in agreement that the benefits of the vaccine outweighed its risks in people aged 18 and older. The vote came after seven hours of debate over technical details of the company’s study and follow-up plans.