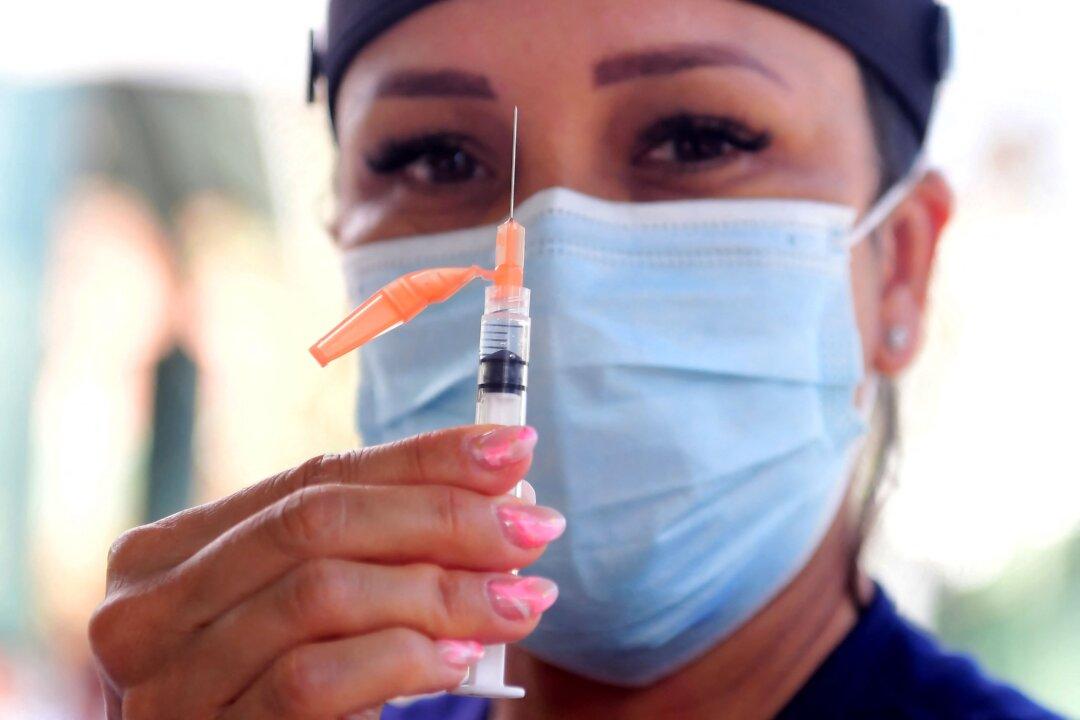
Every adult 18 and older in the United States who received the single-shot Johnson & Johnson COVID-19 vaccine will be able to get a booster dose if the Food and Drug Administration (FDA) accepts a recommendation from its advisory panel.
The panel unanimously voted Friday to advise the agency to authorize boosters for all adults, regardless of their health condition.