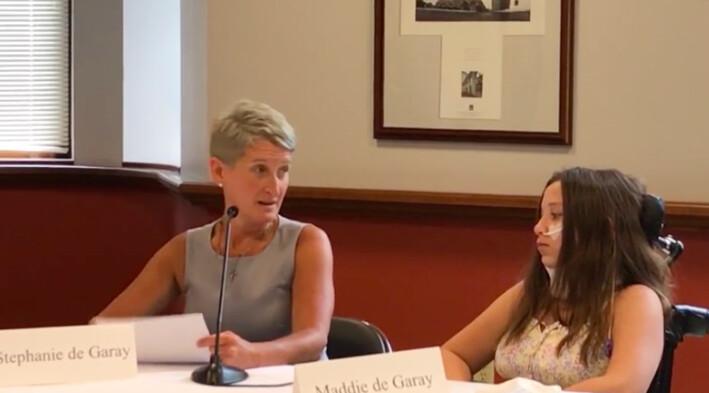Maddie de Garay was a healthy, happy, straight-A student from Ohio who enjoyed hanging out with friends before she received her second dose of the Pfizer COVID-19 vaccine at the age of 12.
The now 13-year-old was one of the 2,260 voluntary participants in the Pfizer COVID-19 vaccine trial for adolescents aged 12 to 15 that began in July 2020. Maddie’s mother, Stephanie de Garay, said her daughter asked to participate in the trial as a way to help end the pandemic.