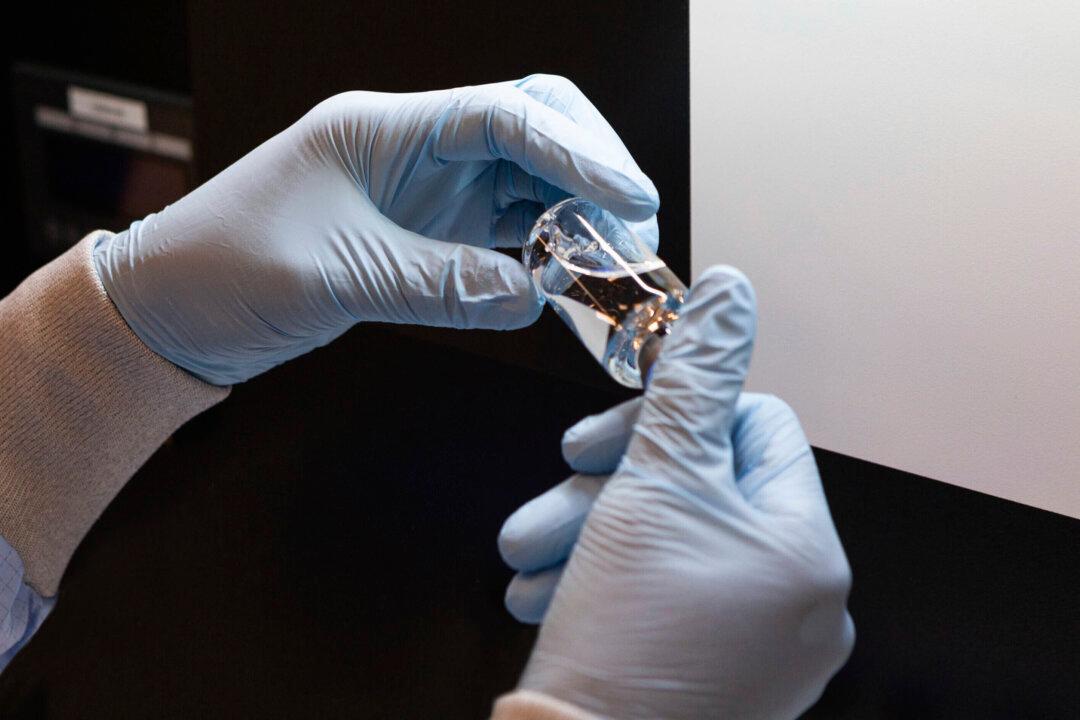
An experimental drug recently touted for its effectiveness against COVID-19 showed a limited benefit for more moderately ill patients, a new late-stage trial showed.
A phase 3 trial found that patients who received remdesivir plus standard of care for five days were 65 percent more likely to have clinical improvement at day 11, versus those with just standard of care. But the few datapoints released showed only nine more patients showed a two or more point improvement on an ordinal scale versus standard of care.