Focus
Emergency Use Authorization
LATEST
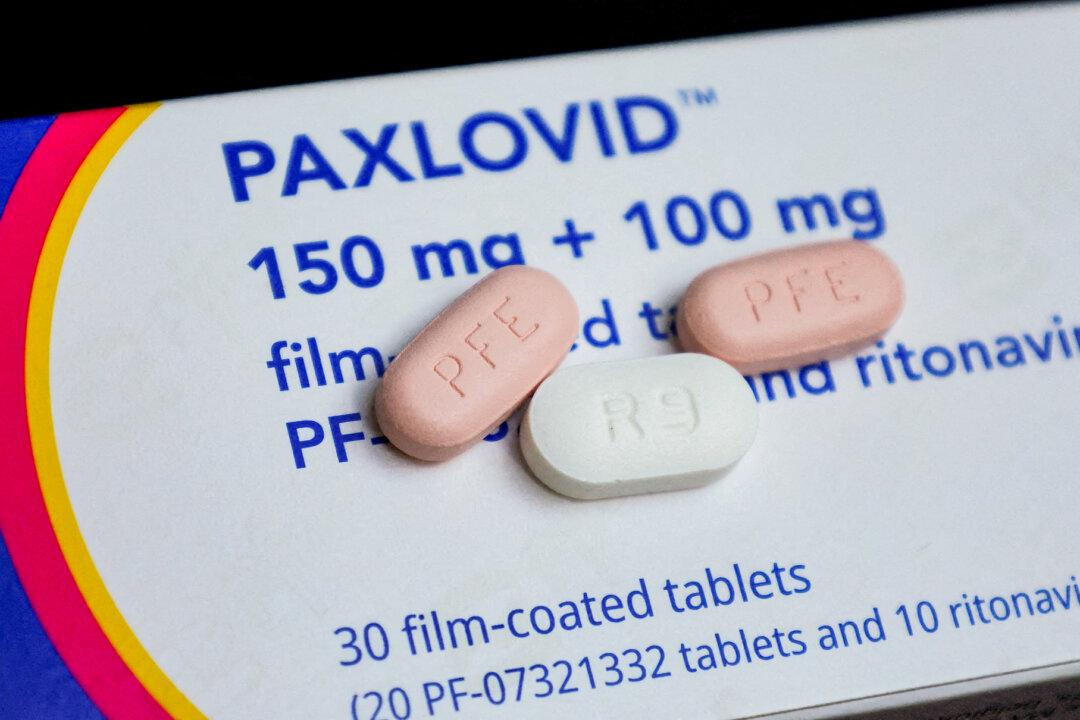
FDA Revises Emergency Use of Paxlovid
The revision is a step toward finalizing the transition from Paxlovid’s emergency use authorization to its new drug application label, initiated in late 2023.
|
|
FDA Revises Emergency Use of Paxlovid
The revision is a step toward finalizing the transition from Paxlovid’s emergency use authorization to its new drug application label, initiated in late 2023.
|
|