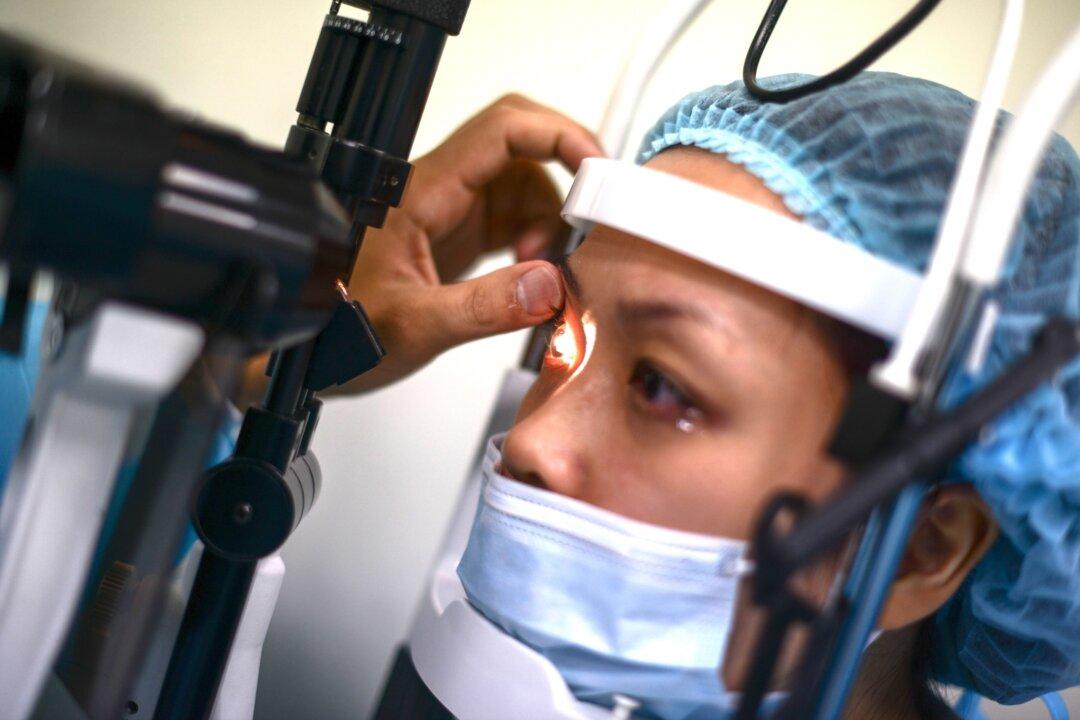
Nearly 25 years after approving LASIK eye surgery as a safe way to correct vision, the U.S. Food and Drug Administration (FDA) is saying that Americans should be better informed about the potential risks associated with the widely popular procedure.
LASIK stands for Laser-Assisted In Situ Keratomileusis. The procedure uses a cutting laser to permanently reshape the cornea—a dome-shaped clear tissue covering the front of the eye—allowing light to focus on the retina at the back of the eye without the help of eyeglasses or contact lenses.