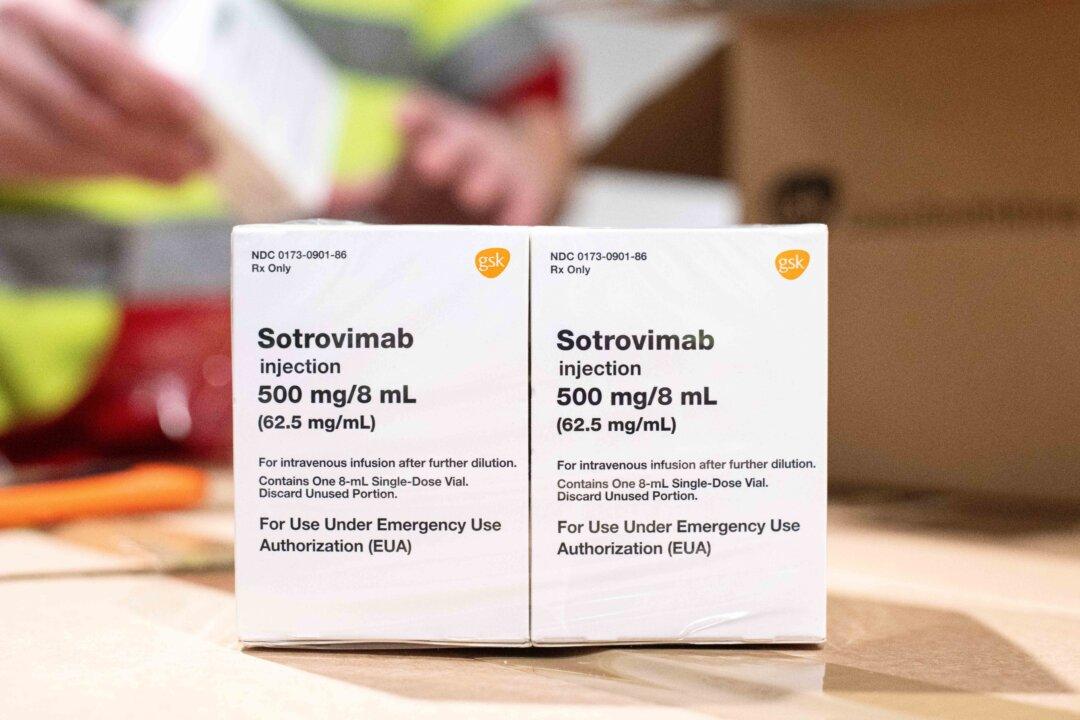
U.S. drug regulators have shifted language on two monoclonal antibody medicines because of emerging subvariants of the Omicron virus variant.
The Food and Drug Administration (FDA) in recent days stipulated that an antibody treatment from GlaxoSmithKline and Vir Biotechnology cannot be used in regions where COVID-19 cases are likely to be caused by a variant “non-susceptible” to the drug.