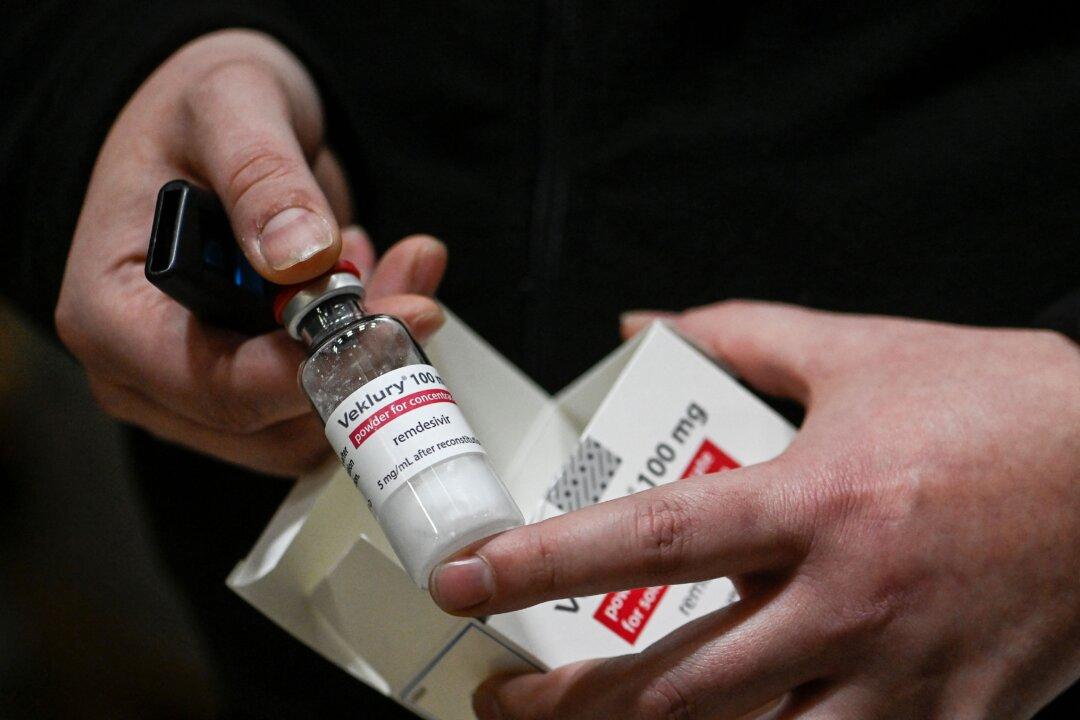
The U.S. Food and Drug Administration (FDA) on April 25 expanded approval of the first COVID-19 treatment for very young children.
The agency “took two actions” to expand the use of Veklury, or remdesivir, to include pediatric patients who are 28 days and older, weigh at least 7 pounds, and have tested positive for COVID-19. The FDA approval is applicable to children who are hospitalized or have mild-to-moderate COVID-19 symptoms and are also at high risk of severe COVID-19.