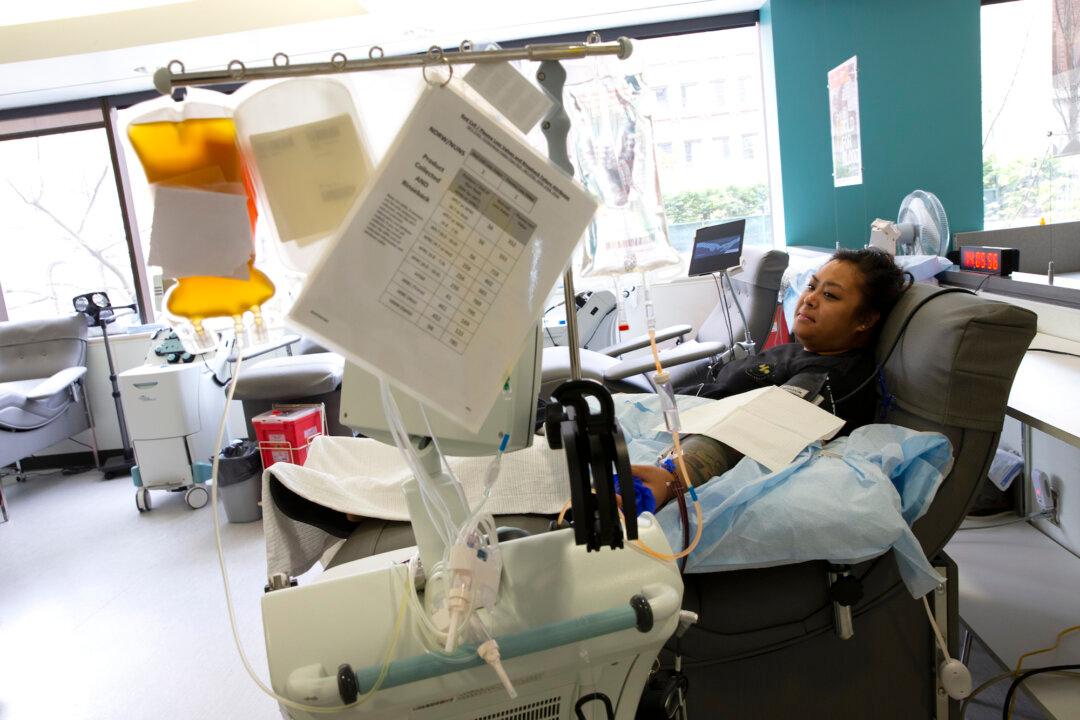
A new study shows that an experimental procedure that uses convalescent plasma—essentially blood from recovered patients—to treat COVID-19 is safe.
Researchers from Mayo Clinic, Michigan State University, and Johns Hopkins University, tracked 5,000 patients who received convalescent plasma transfusions and found few side effects and low mortality in those receiving the experimental treatment.