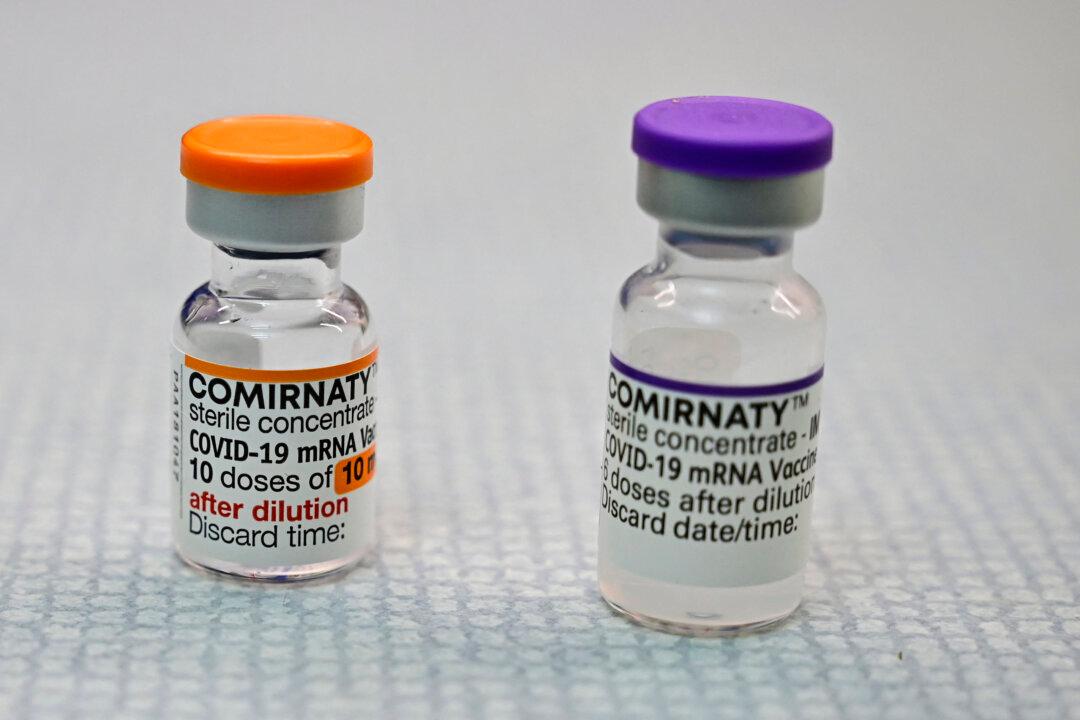
A small number of Comirnaty and Spikevax COVID-19 vaccine doses have become available in the United States in recent weeks, according to court filings and U.S. health departments.
Vials of vaccines labeled Comirnaty started being available to members of the U.S. military in May and tens of thousands of the vials have since been ordered, according to military officials. Dozens of vials were spotted at a clinic in Alaska in June, according to a Coast Guard officer.