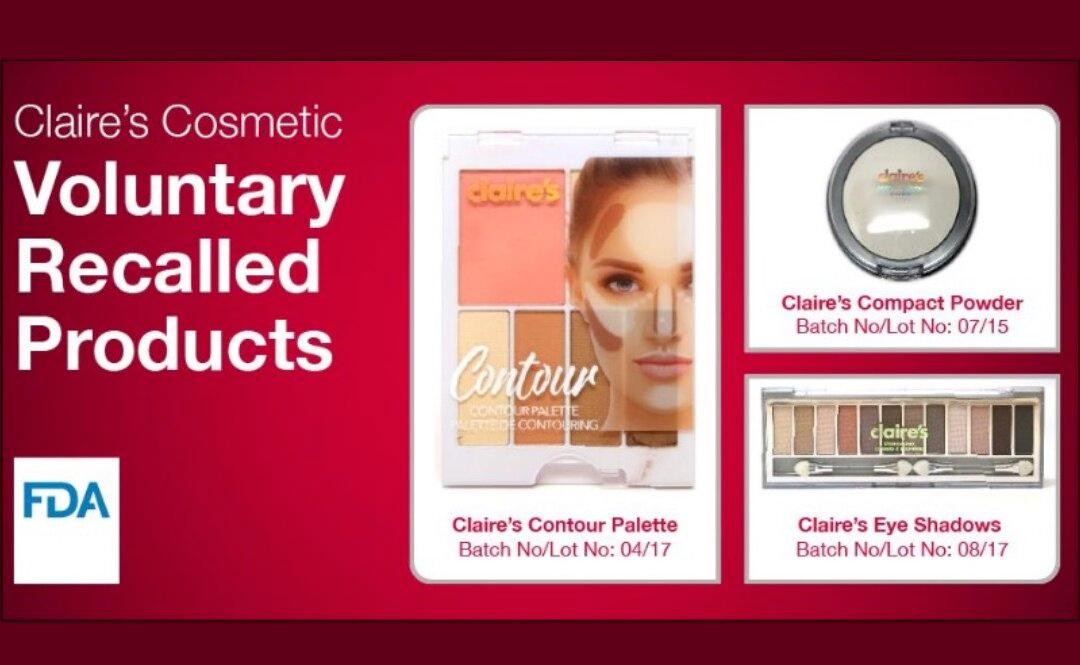
The Food and Drug Administration has advised people to stop using certain lines of Claire’s makeup products after tests showed they may contain asbestos.
Officials said in a March 12 advisory that people should not use Claire’s eye shadows, compact powder, and contour palette “because they may be contaminated with asbestos fibers.”