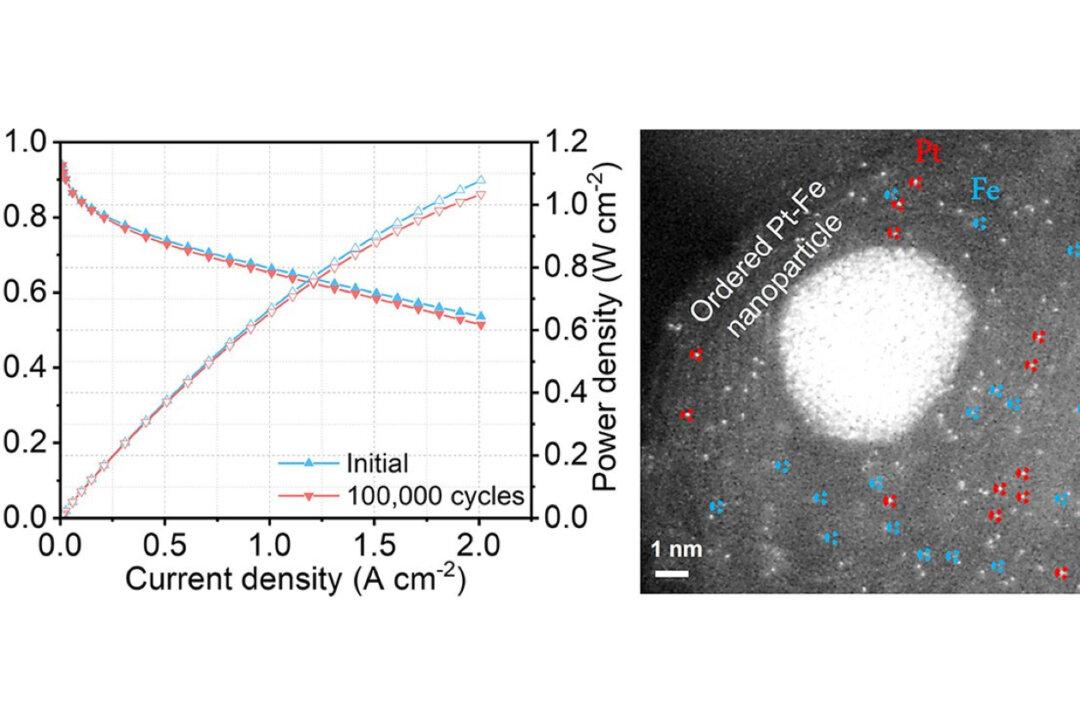
The Hong Kong University of Science and Technology (HKUST) research team has developed a new hydrogen fuel cell, which not only sets a new battery durability record, but it is also more cost-effective, helping to promote the popularity of green energy and to help in achieving the carbon neutral goal.
Hydrogen fuel cells, which use hydrogen and oxygen to generate electricity, are regarded as a cleaner source of electricity because carbon dioxide, suspended particles, and other smog and air pollutants that may cause environmental problems, are not generated during the power generating process.