Scientists with Oxford University say a CCP virus vaccine candidate they’re developing could be available by the fall of 2020.
Most groups working on vaccines have pinpointed early 2021 as the earliest they'll be available for distribution.
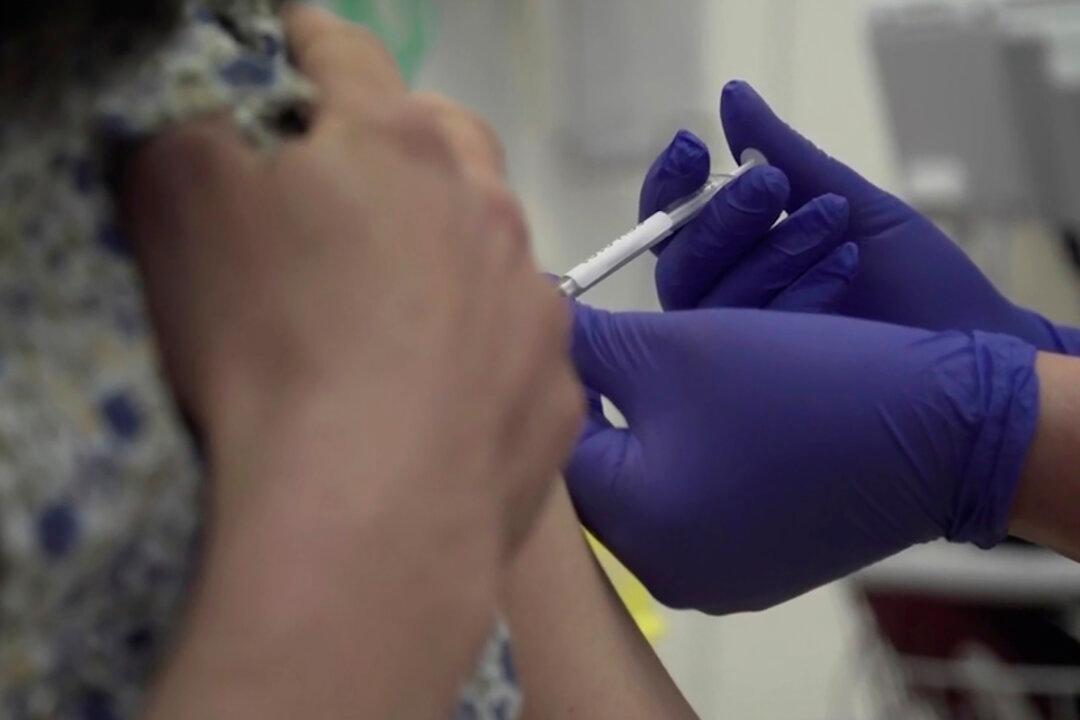

Scientists with Oxford University say a CCP virus vaccine candidate they’re developing could be available by the fall of 2020.
Most groups working on vaccines have pinpointed early 2021 as the earliest they'll be available for distribution.