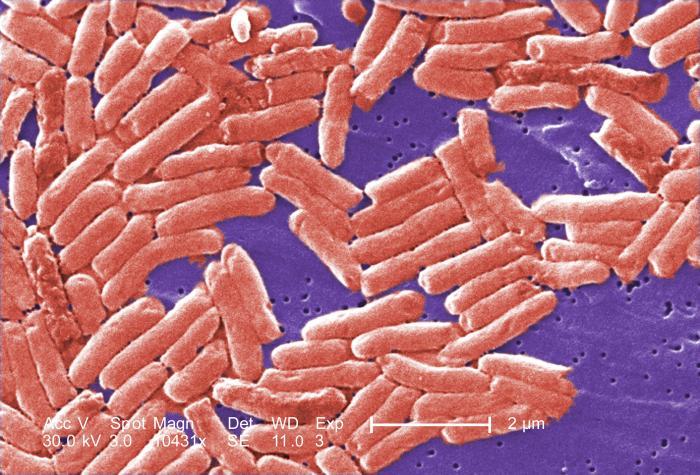
Health care company Abbott Nutrition has announced the recall of multiple baby powder formulas after reports of four infants getting sick through consumption. Certain EleCare, Similac, and Alimentum formulas, part of the recalled products, were manufactured in the company’s Sturgis plant in Michigan.
To identify a recalled product, customers can check the multi-digit number at the bottom of the product container. If it begins with any number between 22 and 37, and contains codes SH, Z2, or K8, it falls under the recall category, given the expiration date is April 1, 2022, or later. None of the products manufactured in other plants are affected by the recall.